- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
HCQ (Hydroxychloroquine) Sulfate Autophagy inhibitor
Cat.No.S4430
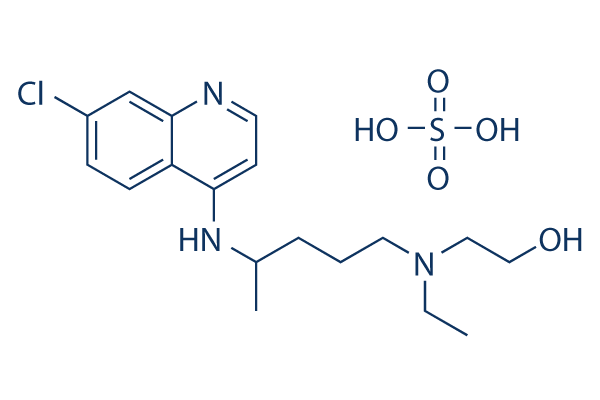
Chemical Structure
Molecular Weight: 433.95
Jump to
Quality Control
Batch:
Purity:
99.86%
99.86
| Related Targets | CXCR Nrf2 Mitophagy LRRK2 ULK FKBP Heme Oxygenase cGAS LC3 Cell wall |
|---|---|
| Other Autophagy Inhibitors | Resveratrol (trans-Resveratrol) Spautin-1 PIK-III DC661 Lys05 Autophinib SMER28 EAD1 Flubendazole EN6 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Function assay | HEK-Blue cells | Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometric analysis, IC50 = 0.11 μM. | 30292896 | |||
| Antiviral assay | Vero E6 cells | 48 h | IC50 for antiviral activity against SARS-CoV-2 in the Vero E6 cell line at 48 h by immunofluorescence-based assay (detecting the viral NP protein in the nucleus of the Vero E6 cells)., IC50 = 0.67608 μM. | 32353859 | ||
| Function assay | HEK-Blue cells | Antagonist activity at human TLR7 expressed in HEK-Blue cells assessed as reduction in CL264-induced NF-kappaB levels after 24 hrs by spectrophotometric analysis, IC50 = 0.8 μM. | 30292896 | |||
| Function assay | HEK293 cells | hERG binding assays: Displacement of [3H]-Dofetilide (5 nM final) from hERG membranes obtained from HEK293 cells, Ki = 2.51189 μM. | 32353859 | |||
| Antiviral assay | Vero E6 cells | IC90 for antiviral activity against SARS-CoV-2 in the Vero E6 cell line by measuring infectious viral titer of supernatent from compound-treated Vero E6 cells by Median Tissue Culture Infectious Dose (TCID)50 by the method of Reed and Muench, IC90 = 5.78 μM. | 32353859 | |||
| Antiproliferative assay | BxPC3 cells | 72 hrs | Antiproliferative activity against human BxPC3 cells after 72 hrs by SRB method, IC50 = 33 μM. | 25699157 | ||
| qHTS assay | A673 cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| qHTS assay | BT-37 cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| qHTS assay | SK-N-MC cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| qHTS assay | NB-EBc1 cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| qHTS assay | LAN-5 cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Antiproliferative assay | A549 cells | 25 ug/ml | 20 hrs | Antiproliferative activity in human A549 cells at 25 ug/ml up to 20 hrs by xCELLigence RTCA SP based cellular impedance analysis | 28570977 | |
| Antiproliferative assay | A549 cells | 25 ug/ml | 60 hrs | Antiproliferative activity in human A549 cells at 25 ug/ml after 60 hrs by xCELLigence RTCA SP based cellular impedance analysis | 28570977 | |
| Autophagy assay | NCI-H3122 cells | 25 to 50 uM | 6 hrs | Inhibition of autophagy in human NCI-H3122 cells assessed as increase in punctate LC3 expression at 25 to 50 uM after 6 hrs by fluorescence microscopic analysis | 25699157 | |
| Apoptosis assay | H460 cells | 25 to 75 uM | 24 hrs | Induction of apoptosis in human H460 cells at 25 to 75 uM after 24 hrs by annexin-V staining-based flow cytometry | 25699157 | |
| Autophagy assay | H460 cells | 24 hrs | Inhibition of autophagy in human H460 cells assessed as increase in LC3-2 level at IC50 after 24 hrs by immunoblot analysis | 25699157 | ||
| Antiviral assay | Vero E6 cells | 2 days | Antiviral efficacy against SARS-CoV-2 (strain BavPat1) in Vero E6 cells assessed by inhibition of viral RNA replication measured by RT-PCR after 2 days, EC50 = 4.17 μM. | ChEMBL | ||
| Antiviral assay | Vero E6 cells | 3 days | IC50 determination at MOI 0.004 using CellTiter- Glo (CTG) assay, performed 3 days post-infection in SARS-CoV-2 infected Vero E6 cells, IC50 = 9.21 μM. | ChEMBL | ||
| Antiviral assay | Vero E6 cells | 3 days | IC50 determination at MOI 0.01 using CellTiter- Glo (CTG) assay, performed 3 days post-infection in SARS-CoV-2 infected Vero E6 cells, IC50 = 11.17 μM. | ChEMBL | ||
| Antiviral assay | Vero E6 cells | 2 days | Antiviral efficacy against SARS-CoV-2 (strain BavPat1) in Vero E6 cells assessed by inhibition of viral RNA replication measured by RT-PCR after 2 days, EC90 = 25.49 μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
Water : 87 mg/mL
DMSO
: Insoluble
Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 433.95 | Formula | C18H28ClN3O5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 747-36-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC 4375 | Smiles | CCN(CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl)CCO.OS(=O)(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
TLR9
Autophagy
|
|---|---|
| In vitro |
Hydroxychloroquine Sulfate is a potent inhibitor of autophagy. It prevents lysosomal acidification, thereby interfering with a key step in the autophagic process.HCQ treatment inhibits RCC (renal cell cancer) cell growth, promotes apoptosis, inhibits mitochondrial oxygen consumption, and increases rates of glycolysis. |
| Kinase Assay |
In vitro kinase assays
|
|
with purified proteins, recombinant S6 protein and recombinant active P70S6K are incubated in 1x kinase buffer with various amount of HCQ or RAD001 in the presence (25 μM) or absence of ATP for 30 minutes at 30°C. Total and phosphorylated S6 at ser235/236 and ser240/244 are detected by western analysis using phosphospecific antibodies. Note that recombinant GST-tagged S6 (53 kd) is distinguished from endogenous S6 (32 kd) on the western blot.
|
|
| In vivo |
The treatment of Hydroxychloroquine Sulfate reduces the infarct size in an in vivo rat model of I/R injury and the cardioprotective effect of Hydroxychloroquine is ERK1/2 dependent. In addition, Hydroxychloroquine Sulfate shows an early vascular protective effect. HCQ seems to prevent the occurrence of endothelial dysfunction(ED) in treated animals. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | NOX2 / β-actin p-NF-κB / β-actin NLRP3 / β-actin p62 / LC3-I / LC3-II / GAPDH |
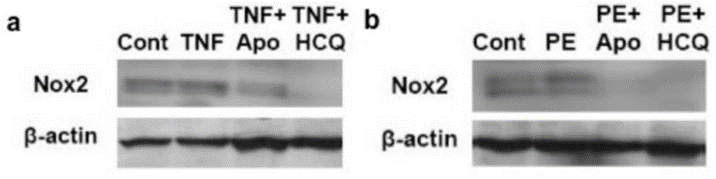
|
32260307 |
| IHC | HE staining of spleen tissue |

|
29456648 |
| Immunofluorescence | ZO-1 |
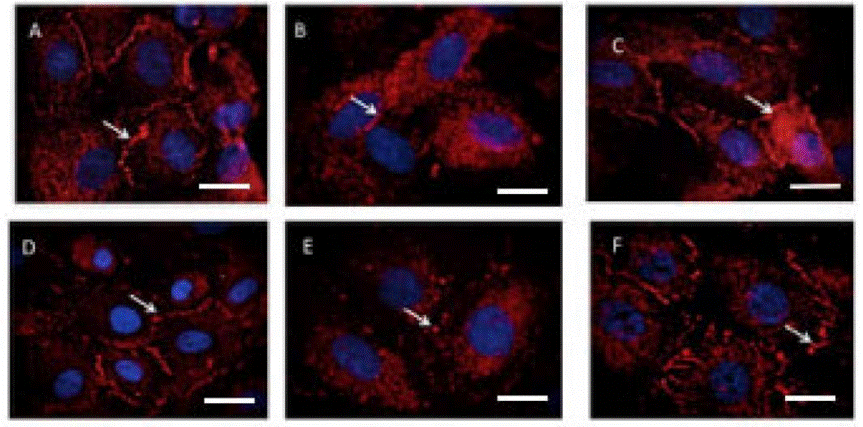
|
32260307 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06408298 | Not yet recruiting | Resectable Localized Prostate Cancer |
Lionel.D.Lewis MD|Dartmouth Cancer Center|Dartmouth-Hitchcock Medical Center |
June 2024 | Early Phase 1 |
| NCT05841758 | Not yet recruiting | Sarcoidosis Pulmonary |
Hospices Civils de Lyon |
April 1 2024 | Phase 4 |
| NCT04731051 | Withdrawn | 2019 Novel Coronavirus |
King Hussein Cancer Center|Amman Pharmaceutical Industries (API)|Sana Pharmaceutical Industry|ACDIMA Biocenter |
October 2022 | Phase 1|Phase 2 |
| NCT05733897 | Recruiting | Nonalcoholic Steatohepatitis |
National Taiwan University Hospital|National Taiwan University |
June 10 2022 | -- |
| NCT05237843 | Unknown status | Recurrent Pregnancy Loss |
Ain Shams University |
March 1 2022 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































