- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
PHA-665752
c-Met inhibitor
research use only
PHA-665752 is a potent, selective and ATP-competitive c-Met inhibitor with IC50 of 9 nM in cell-free assays, >50-fold selectivity for c-Met than RTKs or STKs.
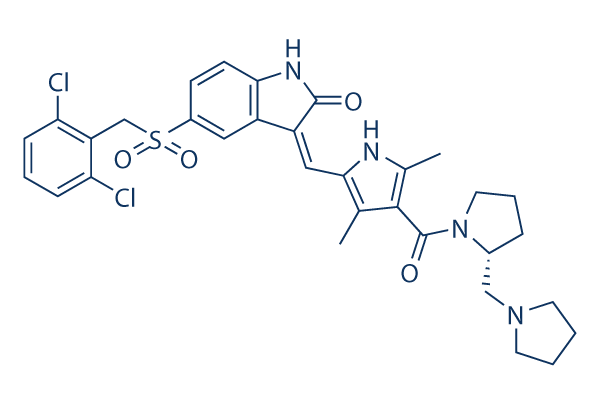
Chemical Structure
Molecular Weight: 641.61
Purity & Quality Control
Batch:
Purity:
99.91%
99.91
Related Products
| Related Products | SGX-523 Foretinib SU11274 BMS-777607 JNJ-38877605 Tivantinib PF-04217903 Tepotinib Savolitinib (AZD6094) MGCD-265 analog Golvatinib (E7050) Merestinib (LY2801653) MK-2461 NPS-1034 AMG 337 Sodium L-ascorbyl-2-phosphate NVP-BVU972 BMS-794833 S49076 | Click to Expand |
|---|---|---|
| Related Compound Libraries | Kinase Inhibitor Library Tyrosine Kinase Inhibitor Library PI3K/Akt Inhibitor Library Cell Cycle compound library Angiogenesis Related compound Library | Click to Expand |
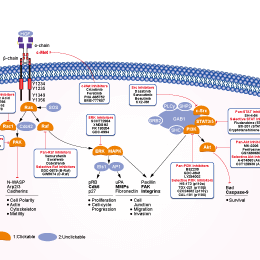
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| NCI-SNU-5 | Growth Inhibition Assay | IC50=0.12375 μM | ||||
| LB2241-RCC | Growth Inhibition Assay | IC50=0.15702 μM | ||||
| KINGS-1 | Growth Inhibition Assay | IC50=0.35911 μM | ||||
| ALL-PO | Growth Inhibition Assay | IC50=0.81277 μM | ||||
| SK-LMS-1 | Growth Inhibition Assay | IC50=0.89846 μM | ||||
| MV-4-11 | Growth Inhibition Assay | IC50=1.2947 μM | ||||
| SUP-T1 | Growth Inhibition Assay | IC50=2.13964 μM | ||||
| MRK-nu-1 | Growth Inhibition Assay | IC50=2.40056 μM | ||||
| ES1 | Growth Inhibition Assay | IC50=3.34866 μM | ||||
| NOS-1 | Growth Inhibition Assay | IC50=4.39867 μM | ||||
| KM12 | Growth Inhibition Assay | IC50=4.418 μM | ||||
| Becker | Growth Inhibition Assay | IC50=5.2466 μM | ||||
| NCI-SNU-1 | Growth Inhibition Assay | IC50=5.63733 μM | ||||
| EW-22 | Growth Inhibition Assay | IC50=7.73614 μM | ||||
| ES6 | Growth Inhibition Assay | IC50=7.8195 μM | ||||
| A498 | Growth Inhibition Assay | IC50=8.28446 μM | ||||
| EW-16 | Growth Inhibition Assay | IC50=9.6543 μM | ||||
| CTV-1 | Growth Inhibition Assay | IC50=9.85024 μM | ||||
| ETK-1 | Growth Inhibition Assay | IC50=10.2931 μM | ||||
| NCI-H1395 | Growth Inhibition Assay | IC50=10.8024 μM | ||||
| DOHH-2 | Growth Inhibition Assay | IC50=10.9264 μM | ||||
| GI-1 | Growth Inhibition Assay | IC50=11.8596 μM | ||||
| HT-144 | Growth Inhibition Assay | IC50=14.2163 μM | ||||
| ES5 | Growth Inhibition Assay | IC50=14.467 μM | ||||
| NALM-6 | Growth Inhibition Assay | IC50=15.2196 μM | ||||
| KNS-81-FD | Growth Inhibition Assay | IC50=15.5849 μM | ||||
| TE-15 | Growth Inhibition Assay | IC50=16.5771 μM | ||||
| SCC-15 | Growth Inhibition Assay | IC50=18.3498 μM | ||||
| EoL-1-cell | Growth Inhibition Assay | IC50=18.4545 μM | ||||
| NCI-H720 | Growth Inhibition Assay | IC50=18.771 μM | ||||
| NB14 | Growth Inhibition Assay | IC50=19.5425 μM | ||||
| KE-37 | Growth Inhibition Assay | IC50=19.8233 μM | ||||
| LXF-289 | Growth Inhibition Assay | IC50=19.8629 μM | ||||
| RPMI-8402 | Growth Inhibition Assay | IC50=20.3269 μM | ||||
| SK-N-DZ | Growth Inhibition Assay | IC50=21.2131 μM | ||||
| ACN | Growth Inhibition Assay | IC50=22.2497 μM | ||||
| TE-11 | Growth Inhibition Assay | IC50=26.069 μM | ||||
| COLO-800 | Growth Inhibition Assay | IC50=27.17 μM | ||||
| MOLT-13 | Growth Inhibition Assay | IC50=27.1847 μM | ||||
| 697 | Growth Inhibition Assay | IC50=28.7633 μM | ||||
| VA-ES-BJ | Growth Inhibition Assay | IC50=29.3729 μM | ||||
| EW-13 | Growth Inhibition Assay | IC50=29.5045 μM | ||||
| NB7 | Growth Inhibition Assay | IC50=32.2665 μM | ||||
| MONO-MAC-6 | Growth Inhibition Assay | IC50=32.8795 μM | ||||
| SW962 | Growth Inhibition Assay | IC50=33.4513 μM | ||||
| KS-1 | Growth Inhibition Assay | IC50=33.9481 μM | ||||
| KU812 | Growth Inhibition Assay | IC50=34.5702 μM | ||||
| IMR-5 | Growth Inhibition Assay | IC50=37.4318 μM | ||||
| BC-1 | Growth Inhibition Assay | IC50=38.033 μM | ||||
| NCI-H510A | Growth Inhibition Assay | IC50=38.2032 μM | ||||
| EW-18 | Growth Inhibition Assay | IC50=40.8303 μM | ||||
| CCRF-CEM | Growth Inhibition Assay | IC50=42.2797 μM | ||||
| HH | Growth Inhibition Assay | IC50=43.5069 μM | ||||
| NCI-H2171 | Growth Inhibition Assay | IC50=46.0272 μM | ||||
| LC-2-ad | Growth Inhibition Assay | IC50=49.1413 μM | ||||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Targets |
|
|---|
In vitro |
||||
| In vitro | PHA-665752 significantly inhibits c-Met kinase activity with Ki of 4 nM, and exhibits >50-fold selectivity for c-Met compared with various tyrosine and serine-threonine kinases. PHA-665752 potently inhibits the HGF-stimulated c-Met autophosphorylation with IC50 of 25-50 nM. PHA-665752 also significantly blocks HGF- and c-Met-dependent functions such as cell motility and cell proliferation with IC50 of 40-50 nM and 18-42 nM, respectively. In addition, PHA-665752 potently inhibits HGF-stimulated or constitutive phosphorylation of mediators of downstream of c-Met such as Gab-1, ERK, Akt, STAT3, PLC-γ, and FAK in multiple tumor cell lines. [1] PHA-665752 inhibits cell growth in TPR-MET-transformed BaF3 cells with IC50 of <60 nM, and inhibits constitutive cell motility and migration by 92.5% at 0.2 μM. Inhibition of c-Met by PHA665752 (0.2 μM) also induces cell apoptosis of 33.1% and G1 cell cycle arrest with cells in G1 phase increasing from 42.4% to 77.0%. PHA665752 can cooperate with rapamycin to inhibit cell growth of TPR-MET-transformed BaF3 cells and non-small cell lung cancer H441 cells. [2] | |||
|---|---|---|---|---|
| Kinase Assay | In vitro enzyme assay | |||
| The c-Met kinase domain GST-fusion protein is used for the c-Met assay. The IC50 value of PHA-665752 for the inhibition of c-Met is based on phosphorylation of kinase peptide substrates or poly-glu-tyr in the presence of ATP and divalent cation (MgCl2 or MnCl2 10-20 mM). The linear range (i.e., the time period over which the rate remains equivalent to the initial rate) is determined for c-Met, and the kinetic measurement and IC50 determination are performed within this range. | ||||
| Cell Research | Cell lines | S114, GTL-16, NCI-H441, and BxPC-3 | ||
| Concentrations | Dissolved in DMSO, final concentrations ~10 μM | |||
| Incubation Time | 18, or 72 hours | |||
| Method | For proliferation assays, cells are grown in medium with 0.1% FBS for 48 hours after which they are treated with various concentrations of PHA-665752 in HGF (50 ng/mL) in a medium containing 2% FBS. After 18 hours, cells are incubated with BrdUrd for 1 hour, fixed, and stained with anti-BrdUrd peroxidase-conjugated antibody, and plates are read at 630 nm. For apoptosis assays, cells are grown in medium with 2% FBS in presence and absence of HGF (50 ng/mL) and various concentrations of PHA-665752 for 72 hours. After 72 hours, a mixture containing ethidium bromide and acridine orange is added, and apoptotic cells (bright orange cells or cell fragments) are counted by fluorescence microscopy. | |||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | IDO p-Met / Met / p-Akt / Akt / p-ERK / ERK |
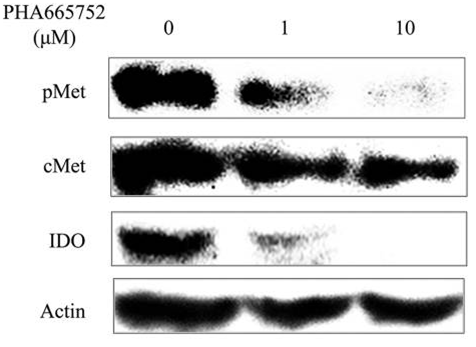
|
27082119 | |
| Growth inhibition assay | Cell proliferation |
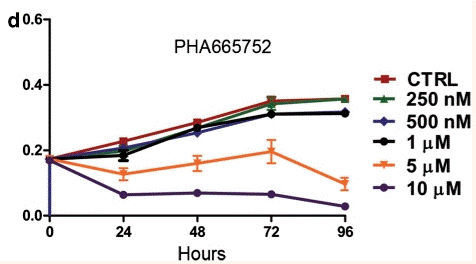
|
20603611 | |
In Vivo |
||
| In vivo | Administration of PHA-665752 induces a dose-dependent tumor growth inhibition of S114 xenografts by 20 %, 39% and 68%, at dose of 7.5, 15, and 30 mg/kg/day, respectively. [1] PHA665752 treatment significantly reduces the tumor growth of NCI-H69, NCI-H441 and A549 in mouse xenografts by 99%, 75%, and 59%, respectively. PHA665752 also significantly inhibits angiogenesis by >85%, due to decreasing the production of vascular endothelial growth factor and increasing the production of the angiogenesis inhibitor thrombospondin-1. [3] | |
|---|---|---|
| Animal Research | Animal Models | Female athymic mice (nu/nu) bearing S114 or GTL-16 tumor xenografts |
| Dosages | ~30 mg/kg/day | |
| Administration | Injection via bolus i.v. | |
References |
|
Chemical Information
| Molecular Weight | 641.61 | Formula | C32H34Cl2N4O4S |
| CAS No. | 477575-56-7 | SDF | Download SDF |
| Synonyms | N/A | ||
| Smiles | CC1=C(NC(=C1C(=O)N2CCCC2CN3CCCC3)C)C=C4C5=C(C=CC(=C5)S(=O)(=O)CC6=C(C=CC=C6Cl)Cl)NC4=O | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 128 mg/mL ( (199.49 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 10 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Frequently Asked Questions
Question 1:
I need to use S1070 for intraperitoneal application in mice. Could you tell me the solvent you use, please?
Answer:
The highest concentration of PHA-665752 (S1070) in 4% DMSO+30% PEG 300+5% Tween 80+ddH2O is 5mg/ml. If you want to get higher concentration, the concentration of DMSO and PEG will be higher. For example, it can be dissolved in 8% DMSO+50% PEG 300+5% Tween 80+ddH2O at 10mg/ml clearly.