- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Ketoconazole
P450 (e.g. CYP17) inhibitor
research use only
Ketoconazole inhibits cyclosporine oxidase and testosterone 6 beta-hydroxylase with IC50 of 0.19 mM and 0.22 mM, respectively. Ketoconazole is an androgen biosynthesis inhibitor.
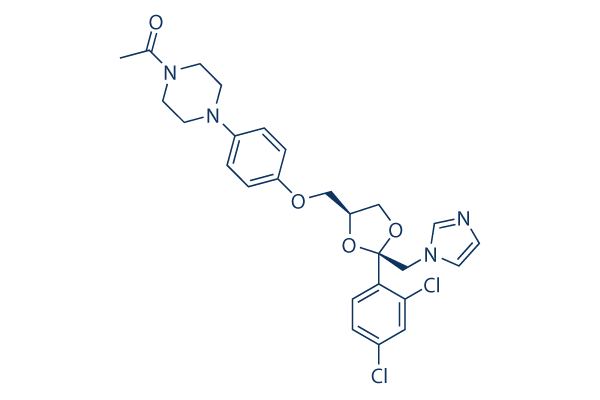
Ketoconazole Chemical Structure
Molecular Weight: 531.43
Purity & Quality Control
Batch:
Purity:
99.95%
99.95
Ketoconazole Related Products
| Related Targets | P450 CYP2 CYP51 CYP3 CYP735 CYP11 CYP26 CYP2C9 CYP1 CYP17 | Click to Expand |
|---|---|---|
| Related Products | Apigenin Baicalein Avasimibe Naringenin Diosmetin Alizarin Sodium Danshensu Orteronel Amentoflavone Tetrahydrocurcumin Uniconazole (S 3307D) Naringin Ellipticine hydrochloride Tanshinone IIA sulfonate sodium Benzbromarone Piperine | Click to Expand |
| Related Compound Libraries | Metabolism Compound Library Anti-cancer Metabolism Compound Library Glutamine Metabolism Compound Library Carbohydrate Metabolism Compound Library Lipid Metabolism Compound Library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| LLC-PK1 epithelial cells | Function assay | Inhibition of P-glycoprotein, human L-MDR1 expressed in LLC-PK1 epithelial cells using calcein-AM polarisation assay, IC50=4.8 μM | ||||
| MCF7 cells | Function assay | Inhibition of CYP26A1 in human MCF7 cells assessed as all-trans retinoic acid metabolism, IC50=12 μM | ||||
| human THP1 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human THP1 cells after 48 hrs, IC50=44 μM | |||
| CHO cells | Function assay | Inhibition of CYP24A1 expressed in CHO cells, IC50=0.52 μM | ||||
| P815B cells | Cytotoxicity assay | 24 h | Cytotoxicity against mouse P815B cells after 24 hrs by MTS/PMS assay, LD50=25 μM | |||
| V79 11B2 cells | Function assay | Inhibition of human CYP11B2 expressed in V79 11B2 cells, IC50=0.081 μM | ||||
| V79 cells | Function assay | Inhibition of human CYP24 hydroxylase expressed in V79 cells, IC50=0.312 μM | ||||
| hamster V79MZh11B1 cells | Function assay | Inhibition of human CYP11B1 expressed in hamster V79MZh11B1 cells, IC50=0.127 μM | ||||
| hamster V79MZh11B2 cells | Function assay | Inhibition of human CYP11B2 expressed in hamster V79MZh11B2 cells, IC50=0.067 μM | ||||
| CHO cells | Function assay | Inhibition of human ERG expressed in CHO cells by whole cell patch clamp technique, IC50=1.90546 μM | ||||
| V79 11B1 cells | Function assay | Inhibition of human CYP11B1 expressed in V79 11B1 cells, IC50=0.224 μM | ||||
| Topp 3 cells | Function assay | Inhibition of human CYP51 expressed in Topp 3 cells by lanosterol demethylase assay, IC50=0.19 μM | ||||
| V79 cells | Function assay | Inhibition of human CYP24A1 expressed in chinese hamster V79 cells, IC50=0.312 μM | ||||
| V79MZ cells | Function assay | Inhibition of human CYP11B2 expressed in hamster V79MZ cells using 11-deoxycorticosterone substrate, IC50=0.067 μM | ||||
| V79MZh cells | Function assay | Inhibition of human CYP11B2 expressed in hamster V79MZh cells, IC50=0.067 μM | ||||
| human epidermal keratinocytes | Function assay | Inhibition of CYP24A1 in human epidermal keratinocytes, IC50=0.126 μM | ||||
| V79MZh cells | Function assay | Inhibition of human CYP11B1 expressed in hamster V79MZh cells, IC50=0.127 μM | ||||
| Click to View More Cell Line Experimental Data | ||||||
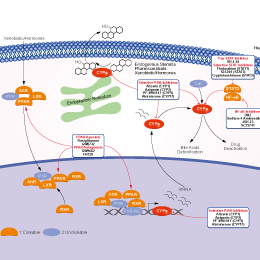
Mechanism of Action
| Description | Ketoconazole inhibits cyclosporine oxidase and testosterone 6 beta-hydroxylase with IC50 of 0.19 mM and 0.22 mM, respectively. Ketoconazole is an androgen biosynthesis inhibitor. | ||||
|---|---|---|---|---|---|
| Features | More active than both Econazole and Miconazole against Malassezia species. | ||||
| Targets |
|
In vitro |
||||
| In vitro | Ketoconazole interacts with androgen receptors in a competitive fashion in intact human foreskin fibroblasts. Ketoconazole competes for [3H]dexamethasone binding to fibroblast glucocorticoid receptors with IC50 of 0.3 mM. [2] Ketoconazole reduces cell proliferation and [3H]thymidine incorporation with IC50 of 2.5 mM in the serum independent HT29-S-B6 colon cell clone. Ketoconazole inhibits the incorporation of [3H]thymidine with IC50 of 2 μM and 13 μM in the Evsa-T cell line and MDA-MB-231 cell line, respectively. Ketoconazole induces a decrease of the number of cells in S phase and a corresponding increase of the percentage of cells in Go-G1 in HT29-S-B6 cells. [3] Ketoconazole is susceptable to several Malassezia species with minimum inhibitory concentrations (MICs) of 0.03 µg/mL. [4] | |||
|---|---|---|---|---|
| Kinase Assay | Whole Cell [3H]R1881 Binding Assay | |||
| Fibroblasts are grown to confluence in five or six 150 cm2 tissue culture flasks for routine assay. This usually requires 4-6 weeks from the time of the initial seeding of the cell line. All studies are performed between passages 3-20. Two days before assay, the medium is changed to one lacking fetal calf serum. This is repeated again 24 hours before assay. Competition assays are performed with 0.5-1.0 nM [3H]R1881 and increasing amounts of the nonradioactive compounds. Binding to low affinity sites is determined in the presence of 5 × 10-7 M R1881 and is subtracted from whole cell binding of [3H]R 1881 obtained in the absence of any inhibitor to assess binding to 5 high affinity site | ||||
| Cell Research | Cell lines | HT29-S-B6 colon cell | ||
| Concentrations | 25 μM | |||
| Incubation Time | 72 hours | |||
| Method | HT29-S-B6 cells (5×105) are plated in 35-mm Petri dishes. The next day, the medium is changed and effectors are added in a small volume (10-20 μL). The incubation medium is renewed every day during the experiments. The same triplicate dishes are used for cell counts, [3H]thymidine incorporation, and flow cytometry. [3H]Thymidine (0.5 μCi) is allowed to incorporate for 24 hours; at the end of incubation, cells are rinsed with 1 mL of medium, detached with 1 mL of trypsin-EDTA, and diluted (1:3) with the culture medium. An aliquot (0.5-1 mL) is used for cell count with a Coulter Counter. | |||
In Vivo |
||
| In vivo | Ketoconazole (25 mg/kg, i.p.) significantly decreases plasma corticosterone and reduces low dose cocaine self-administration without affecting food-reinforced responding in rats. [5] Ketoconazole raises the AUC of orally administered digoxin from 63 mg x h/L to 411 mg x h/L in rats. Ketoconazole raises the AUC of intravenously administered digoxin from 93 mg × h/L to 486 mg × h/L in rats. Ketoconazole increases digoxin bioavailability from 0.68 to 0.84 in rats, while mean absorption time is reduced from 1.1 hours to 0.3 hour. [6] | |
|---|---|---|
| Animal Research | Animal Models | male Wistar rats |
| Dosages | 25 mg/kg | |
| Administration | Intraperitoneal injection | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04869449 | Recruiting | Glioblastoma|Glioblastoma Multiforme|Glioblastoma Multiforme of Brain|Glioblastoma Multiforme Adult |
Milton S. Hershey Medical Center |
May 12 2022 | Early Phase 1 |
| NCT04212000 | Completed | Healthy |
Cortendo AB |
December 16 2019 | Phase 1 |
| NCT03796273 | Recruiting | Anatomic Stage IV Breast Cancer AJCC v8|Astrocytoma|Breast Carcinoma Metastatic in the Brain|Glioma|Invasive Breast Carcinoma|Oligodendroglioma|Prognostic Stage IV Breast Cancer AJCC v8|Recurrent Glioma |
Wake Forest University Health Sciences|National Cancer Institute (NCI) |
March 13 2019 | Early Phase 1 |
| NCT04872920 | Recruiting | Cushing Syndrome |
HRA Pharma |
December 20 2018 | -- |
| NCT03473418 | Unknown status | Vaginal Candidiasis |
Assiut University |
April 1 2018 | Phase 3 |
References |
|
Chemical Information
| Molecular Weight | 531.43 | Formula | C26H28Cl2N4O4 |
| CAS No. | 65277-42-1 | SDF | Download Ketoconazole SDF |
| Synonyms | R 41400 | ||
| Smiles | CC(=O)N1CCN(CC1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=CN=C4)C5=C(C=C(C=C5)Cl)Cl | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
Ethanol : 7 mg/mL DMSO : 3 mg/mL ( (5.64 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field