research use only
Benzbromarone P450 (e.g. CYP17) inhibitor
Cat.No.S4221
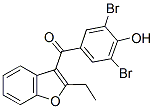
Chemical Structure
Molecular Weight: 424.08
Quality Control
| Related Targets | Dehydrogenase HSP Transferase PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism Drug Metabolite |
|---|---|
| Other P450 (e.g. CYP17) Inhibitors | Apigenin Baicalein Avasimibe Naringenin Diosmetin Sodium Danshensu Alizarin Naringin Orteronel Danshensu |
Solubility
|
In vitro |
DMSO
: 85 mg/mL
(200.43 mM)
Ethanol : 15 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 424.08 | Formula | C17H12Br2O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 3562-84-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Desuric | Smiles | CCC1=C(C2=CC=CC=C2O1)C(=O)C3=CC(=C(C(=C3)Br)O)Br | ||
Mechanism of Action
| Targets/IC50/Ki |
CYP2C9
19.3 nM(Ki)
|
|---|---|
| In vitro |
Benzbromarone (20 μM) decreases mitochondrial membrane potential by 81% in isolated rat hepatocytes. This compound decreases state 3 oxidation and respiratory control ratios for L-glutamate with IC50 < 1 μM in isolated rat liver mitochondria. It (50 μM) uncouples oxidative phosphorylation and increases oxygen consumption by hepatocytes starting at 10 μM in isolated rat hepatocytes. This chemical also inhibits the formation of acid-soluble β-oxidation products in a dose-dependent manner with IC50 of 2 μM. It (100 μM) inhibits the electron transport chain and are uncouplers of oxidative phosphorylation in isolated rat liver mitochondria. This compound (1 μM) leads to concentration-dependent increasion of ROS production in HepG2 cells. It (100 μM) leads to a significant increase in mitochondrial size of isolated rat liver mitochondria. This chemical is associated with leakage of cytochrome c into the cytoplasm of HepG2 cells. It (100 μM) results in the proportion of apoptotic cells of 11% in rat hepatocytes. This compound significantly reduces the oxypurinol uptake at a concentration as low as 10 nM and completely blocks it at 1 μM. It (1 μM) uptakes the typical substrate of OCTN1 (tetraethylammonium) and OCTN2 (carnitine) in the HEK293 cells expressed with human OCTN1 by 96.7% and 111% of control, respectively. This chemical completely inhibits urate uptake at 50 μM in URAT1-expressing oocytes, with IC50 of less than 0.1 μM. It activates through sequential hydroxylation of the benzofuran ring to a catechol, which can then be further oxidized to a reactive quinone intermediate capable of adducting protein. |
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































