research use only
Avasimibe P450 (e.g. CYP17) inhibitor
Cat.No.S2187
Chemical Structure
Molecular Weight: 501.72
Quality Control
| Related Targets | Dehydrogenase HSP Transferase PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism Drug Metabolite |
|---|---|
| Other P450 (e.g. CYP17) Inhibitors | Apigenin Baicalein Naringenin Diosmetin Alizarin Sodium Danshensu Naringin Benzbromarone Orteronel Danshensu |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| THP1 cells | Function assay | Inhibition of ACAT-mediated esterified cholesterol accumulation in human THP1 cells exposed to acetyl-LDL during differentiation assessed as effect on foam cell formation, IC50=1.5 μM | 18620381 | |||
| HepG2 cells | Function assay | Inhibition of ACAT in human HepG2 cells, IC50=0.479 μM | 19167888 | |||
| rat macrophages | Function assay | 24 h | Inhibition of ACAT in rat macrophages assessed as incorporation of extracellular [3H]-oleic acid-BSA complex into the intracellular cholesteryl ester after 24 hrs, IC50=0.479 μM | 19464189 | ||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| IC21 | Function assay | Inhibition of ACAT1 in mouse IC21 cells in absence of BSA, IC50=0.06μM | 29945757 | |||
| J774 | Function assay | 18 hrs | Inhibition of ACAT1 in mouse J774 cells assessed as reduction in esterified cholesterol accumulation after 18 hrs in presence of 25-hydroxycholesterol, IC50=0.59μM | 29945757 | ||
| IC21 | Function assay | Inhibition of ACAT1 in mouse IC21 cells in presence of BSA, IC50=0.76μM | 29945757 | |||
| Caco-2 | Function assay | 48 hrs | Determination of IC50 values for inhibition of SARS-CoV-2 induced cytotoxicity of Caco-2 cells after 48 hours by high content imaging, IC50=3.93μM | ChEMBL | ||
| Caco-2 | Toxicity assay | 48 hrs | Toxicity against Caco-2 cells determined at 48 hours by intracellular ATP concentration using the CellTiter-Glo Luminescent Cell Viability Assay, CC50=13.71μM | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(199.31 mM)
Warmed with 50°C water bath;
Ultrasonicated;
Ethanol : 16 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 501.72 | Formula | C29H43NO4S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 166518-60-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CI-1011, PD-148515 | Smiles | CC(C)C1=C(C(=CC=C1)C(C)C)OS(=O)(=O)NC(=O)CC2=C(C=C(C=C2C(C)C)C(C)C)C(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
CYP2C9
(Human Hepatic Microsomes) 2.9 μM
ACAT
(IC-21 macrophages) 3.3 μM
CYP1A2
(Human Hepatic Microsomes) 13.9 μM
CYP2C19
(Human Hepatic Microsomes) 26.5 μM
|
|---|---|
| In vitro |
Avasimibe at concentration of 1μg/mL causes reduction of Total cholesterol (TC) and Esterified cholesterol (EC) through inhibiting LDL binding and decreasing scavenger receptor numbers during foam cell formation in human monocyte-derived macrophages (HMMs). This compound at concentration of 2μg/mL enhances cholesterol efflux from established HMM foam cells preincubating with 10μg/ml LDL. It inhibits Lipoprotein(a) accumulation in the culture media of primary monkey hepatocyte in a dose-dependent manner with 11.9% -31.3% inhibition, the change is mainly associated with decreased ApoA. This chemical incubating at concentration of 10 nM, 1 μM, and 10 μM for 24 hours in HepG2 cells reduce ApoB secretion into media by 25%, 27%, and 43%, respectively. It decreases ApoB secretion by enhanced intracellular degradation of ApoB rather than decreased synthesis of ApoB. The compound inhibits ACTC with IC50 of 3.3 μM in IC-21 macrophages with consideration of the total inhibitor concentration in the assay sample. It inhibits human P450 isoenzymes CYP2C9, CYP1A2 and CYP2C19 with IC50 of 2.9 μM, 13.9 μM and 26.5 μM, respectively. This inhibitor inhibits ACAT-1 expression and cholesterol ester synthesis in glioma cell lines. It inhibits the growth of the glioma cells by inducing cell cycle arrest and apoptosis due to caspase-8 and caspase-3 activation. |
| Kinase Assay |
P450 Inhibition Studies
|
|
Pooled human liver microsomes (HLM) from at least 15 donors are used for all inhibition assays. For IC50 determinations, the substrate probes are used at their approximate in vitro Km values. All incubations are performed with 100 mM potassium phosphate buffer (pH 7.4) and 1 mM NADPH. For CYP1A2 inhibition study, incubations are performed in a total volume of 0.5 ml, in duplicates with 0.1 mg/ml HLM, 30 μM phenacetin, 1 mM NADPH, and in the presence of this compound (0, 0.3, 0.75, 1.5, 3, 7.5, 15, 30, and 40 μM in 50 mM) in a potassium phosphate buffer at pH 7.4. After preincubation at 37 °C for 7 min, NADPH is added to initiate the enzyme reaction. The reaction mixture is quenched with 500 μl of ice-cold 100 ng/ml paracetamol-D4/CH3CN after 25 min. The standards (4-acetamidophenol, singlet) and quality controls (triplicates for low, medium, and high) are prepared at room temperature. After mixing, 0.2 ml of the samples is transferred to another plate and submitted for LC/MS/MS analysis after centrifugation at 3000 rpm for 10 min. A Supelco Discovery Amide C16, 100 × 2.1 mm (5-μm particle size) column (Supelco, Bellefonte, PA) is used. The mobile phase is isocratic, 40:60 [acetonitrile/formic acid, 0.1% (v/v)] at 0.2 ml/min.
|
|
| In vivo |
Avasimibe significantly reduces Lipoprotein(a) and total cholesterol levels in nine healthy male monkeys with a normal chow diet orally treated with CI-1011 at 30 mg/kg per day for 3 weeks, Lipoprotein(a) and total cholesterol levels reduce to 68 and 73% of control levels, respectively. This compound decreases total cholesterol mainly due to reduction of low density lipoprotein (LDL). It decreases amyloid plaque load in the cortex and hippocampus and reduces the levels of insoluble Abeta40 and Abeta42 and C-terminal fragments of amyloid precursor protein (APP) in brain extracts in young human APP transgenic mice. This chemical reduces diffuse amyloid plaques by suppression of astrogliosis and enhanced microglial activation in aged human APP transgenic mice. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-AKT / AKT Active β-catenin |
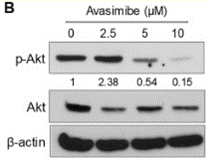
|
29489864 |
| Immunofluorescence | β-catenin |
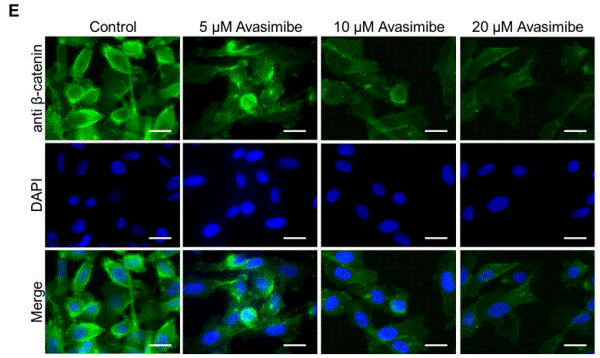
|
29545473 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
I'd like to have more information about the in vivo administration of it in monkeys?
Answer:
It can be dissolved in 2% DMSO+corn oil at 5mg/ml clearly, and in 0.5% CMC Na+1% Tween 80 at 10mg/ml as a suspension.
Question 2:
We want to use it for mice study by IP injection. I was wondering if you have any suggestions how to make a soluble solution for IP injection?
Answer:
S2187 can be dissolved in 5% DMSO+30% PEG 300+5% Tween 80+ddH2O at 1 mg/ml as a clear solution. When preparing the solution, please dissolve it in DMSO clearly first, then add PEG and Tween. After they mixed well, then dilute with water. And we found after stayed for about 20min, the precipitation will go out. So please prepare the solution just before use.






































