- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Abiraterone Acetate
P450 (e.g. CYP17) inhibitor
research use only
Abiraterone Acetate is an acetate salt form of Abiraterone which is a steroidal cytochrome CYP17 inhibitor with IC50 of 72 nM in a cell-free assay. Abiraterone acetate is an oral androgen biosynthesis inhibitor.
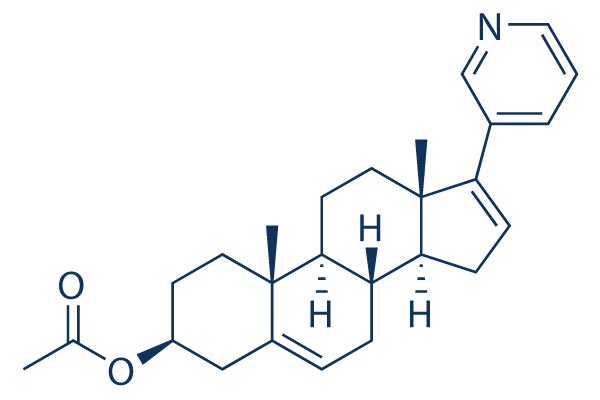
Abiraterone Acetate Chemical Structure
Molecular Weight: 391.55
Purity & Quality Control
Batch:
Purity:
99.98%
99.98
Abiraterone Acetate Related Products
| Related Targets | P450 CYP2 CYP51 CYP3 CYP735 CYP11 CYP26 CYP2C9 CYP1 CYP17 | Click to Expand |
|---|---|---|
| Related Products | Apigenin Baicalein Avasimibe Naringenin Diosmetin Alizarin Sodium Danshensu Orteronel Amentoflavone Tetrahydrocurcumin Uniconazole (S 3307D) Naringin Ellipticine hydrochloride Tanshinone IIA sulfonate sodium Benzbromarone Piperine | Click to Expand |
| Related Compound Libraries | Metabolism Compound Library Anti-cancer Metabolism Compound Library Glutamine Metabolism Compound Library Carbohydrate Metabolism Compound Library Lipid Metabolism Compound Library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| JM109 | Function assay | Inhibition of C-terminal His-tagged recombinant human CYP17A1delta19H mutant expressed in Escherichia coli JM109 cells assessed as decrease in progesterone hydroxylation in presence of cytochrome P450 reductase by HPLC-UV method, IC50 = 0.0094 μM. | 29792703 | |||
| P450c17-LNCaP | Function assay | In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escherichia coli or P450c17-LNCaP cells as the enzyme source, IC50 = 0.8 μM. | 12773039 | |||
| V79MZh11B1 | Function assay | Inhibition of human CYP11B1 expressed in hamster V79MZh11B1 cells, IC50 = 1.608 μM. | 18672868 | |||
| V79MZh11B1 | Function assay | Inhibition of recombinant CYP11B1 expressed in expressed in V79MZh11B1 cells, IC50 = 1.608 μM. | 19211174 | |||
| V79MZh | Function assay | Inhibition of human CYP11B1 expressed in hamster V79MZh cells, IC50 = 1.61 μM. | 20550118 | |||
| V79MZh | Function assay | Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate, IC50 = 1.61 μM. | 23859149 | |||
| V79MZh | Function assay | Inhibition of human CYP11B2 expressed in hamster V79MZh cells, IC50 = 1.75 μM. | 20550118 | |||
| V79MZh | Function assay | Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate, IC50 = 1.75 μM. | 23859149 | |||
| V79MZh11B2 | Function assay | Inhibition of human CYP11B2 expressed in hamster V79MZh11B2 cells, IC50 = 1.751 μM. | 18672868 | |||
| LNCAP | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LNCAP cells after 72 hrs by MTT assay, IC50 = 3.29 μM. | 29310026 | ||
| hTERT-BJ | Cytotoxicity assay | 48 hrs | Cytotoxicity against human hTERT-BJ cells assessed as cell growth inhibition after 48 hrs SRB assay, GI50 = 4.5 μM. | 29172080 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 5.94 μM. | 29310026 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 7.72 μM. | 29310026 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by SRB assay, GI50 = 7.9 μM. | 29172080 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 9.32 μM. | 24148837 | ||
| GES-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GES-1 cells after 72 hrs by MTT assay, IC50 = 13.12 μM. | 29310026 | ||
| T47D | Growth inhibition assay | 72 hrs | Growth inhibition of human T47D cells after 72 hrs by MTT assay, IC50 = 16.9 μM. | 27209562 | ||
| MDA-MB-231 | Growth inhibition assay | 72 hrs | Growth inhibition of human MDA-MB-231 cells after 72 hrs by MTT assay, IC50 = 19.2 μM. | 27209562 | ||
| MCF7 | Growth inhibition assay | 72 hrs | Growth inhibition of human MCF7 cells after 72 hrs by MTT assay, IC50 = 19.3 μM. | 27209562 | ||
| MDA-MB-361 | Growth inhibition assay | 72 hrs | Growth inhibition of human MDA-MB-361 cells after 72 hrs by MTT assay, IC50 = 20.4 μM. | 27209562 | ||
| T47D | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T47D cells after 48 hrs by SRB assay, GI50 = 24 μM. | 29172080 | ||
| WiDr | Antiproliferative assay | 48 hrs | Antiproliferative activity against human WiDr cells after 48 hrs by SRB assay, GI50 = 42 μM. | 29172080 | ||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Description | Abiraterone Acetate is an acetate salt form of Abiraterone which is a steroidal cytochrome CYP17 inhibitor with IC50 of 72 nM in a cell-free assay. Abiraterone acetate is an oral androgen biosynthesis inhibitor. | ||
|---|---|---|---|
| Features | Abiraterone is a drug used in castration-resistant prostate cancer. | ||
| Targets |
|
In vitro |
||||
| In vitro | Abiraterone shows a good complexation with the heme iron only in SM1. [1] Abiraterone blocks the synthesis of androgens by inhibiting CYP17A1. Abiraterone also blocks 3β-hydroxysteroid dehydrogenase (3βHSD), an enzyme that is absolutely required for the synthesis of biologically active androgens. Abiraterone inhibits conversion of DHEA to Δ4-androstenedione. Abiraterone inhibition of 3βHSD blocks DHT synthesis and the androgen receptor response. Abiraterone inhibits the conversion of Δ5-androstenediol to testosterone. [2] Abiraterone inhibits C17,20-lyase, with an IC50 of 5.8 nM, in rat testis microsomes. Abiraterone significantly inhibits testosterone secretion (−48%) and in turn increases LH concentration (192%). [3] Abiraterone inhibits in vitro proliferation and AR-regulated gene expression of AR-positive prostate cancer cells, which could be explained by AR antagonism in addition to inhibition of steroidogenesis. [4] |
|||
|---|---|---|---|---|
| Kinase Assay | C17,20-lyase activity assay | |||
| Microsomes are diluted to a final protein concentration of 50 μg/mL in the reaction mixture which contains 0.25 M sucrose, 20 mM Tris-HCl (pH 7.4), 10 mM G6P and 1.2 IU/mL G6PDH. After equilibration at 37 °C for 10 minutes, the reaction is initiated by addition of βNADP to obtain a final concentration of 0.6 mM. Prior to the distribution of 600 μL of the reaction mixture in each tube, test compounds are evaporated to dryness under a stream of nitrogen and then are incubated at 37 °C for 10 minutes. After incubation with Abiraterone, 500 μL of the reaction mixture is transferred to tubes containing 1 μM of the enzyme substrate, 17OHP. After a further 10 minutes incubation, tubes are placed on ice and the reaction is stopped by addition of 0.1 ml NaOH 1N. Tubes are deep-frozen and stored at -20 °C until assayed for Δ4A levels. A Δ4A RIA is developed and automated on a microplate format in our laboratory using a specific antibody against Δ4A and instructions provided by Biogenesis. The separation of free and bound antigen is achieved with a dextran-coated charcoal suspension. After centrifugation, aliquots of the clear supernatant are counted in duplicates in a liquid scintillation counter. The Δ4A concentrations of unknown samples are determined from the standard curve. The detection limit is 0.5 ng/mL and the within and between assay coefficients of variation are 10.7 and 17.6%, respectively at an assay value of 13 ng/mL. The rate of enzymatic reaction is expressed as pmol of Δ4A formed per 10 minutes and per mg of protein. The value of maximum activity without inhibitor (control) is set at 100%. The IC50 values are calculated using non-linear analysis from the plot of enzyme activity (%) against log of inhibitor concentration. | ||||
| Cell Research | Cell lines | LNCaP and VCaP cells | ||
| Concentrations | 0 μM -10 μM | |||
| Incubation Time | 24 hours and 96 hours | |||
| Method | LNCaP and VCaP cells are seeded in 96-well plates and grown in CSS-supplemented phenol red-free or FBS-supplemented media for 7 days. Cells are treated with Abiraterone at 24 hours and 96 hours after plating and cell viability is determined on day 7 by adding CellTiter Glo and measuring luminescence. |
|||
In Vivo |
||
| In vivo | Following intraperitoneal administration in a rodent model, abiraterone was found to have rapid deacetylation. When administered as its acetate pro-drug (CB 7630), it suppressed circulating testosterone to undetectable levels and markedly decreased the weights of androgen sensitive organs. Abiraterone is well tolerated and the mean elimination half-life of abiraterone in these studies was 27.6 h (thus supporting the use of once-daily dosing)[5]. Preclinical studies with abiraterone demonstrated reduction in androgen production downstream of CYP17 which resulted in decreased weight of the ventral prostate, testis, and seminal vesicles in mice[6]. |
|
|---|---|---|
| Animal Research | Animal Models | Male NOD/SCID mice with LAPC4 cells |
| Dosages | 0.5 mmol/kg/d | |
| Administration | Administered via s.c. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03348670 | Active not recruiting | Prostate Cancer |
Han Xu M.D. Ph.D. FAPCR Sponsor-Investigator IRB Chair|Medicine Invention Design Inc |
August 18 2023 | Phase 2|Phase 3 |
| NCT06014853 | Completed | Prostate Cancer |
Bukwang Pharmaceutical|Dyna Therapeutics |
August 10 2023 | Phase 1 |
| NCT05737082 | Completed | Healthy |
Hanmi Pharmaceutical Company Limited |
March 30 2023 | Phase 1 |
References |
|
Chemical Information
| Molecular Weight | 391.55 | Formula | C26H33NO2 |
| CAS No. | 154229-18-2 | SDF | Download Abiraterone Acetate SDF |
| Synonyms | CB7630 Acetate | ||
| Smiles | CC(=O)OC1CCC2(C3CCC4(C(C3CC=C2C1)CC=C4C5=CN=CC=C5)C)C | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 11 mg/mL ( (28.09 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field