research use only
LY2109761 TGF-beta/Smad inhibitor
Cat.No.S2704
Chemical Structure
Molecular Weight: 441.52
Quality Control
Products Often Used Together with LY2109761
| Related Targets | PKC ROCK Bcr-Abl |
|---|---|
| Other TGF-beta/Smad Inhibitors | SB431542 LDN-193189 Galunisertib (LY2157299) RepSox (E-616452) A-83-01 Vactosertib (TEW-7197) SRI-011381 (C381) SB-525334 LDN-193189 Dihydrochloride SIS3 Hydrochloride |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HepG2 | Function Assay | 10 μM | 2 h | inhibits autophagy induction by galangin | 25268046 | |
| PC-3 | Function Assay | 0.2/2/4 μM | 24 h | DMSO | inhibits TGF-β1–induced Smad2 activation | 22173053 |
| PMOs | Function Assay | 0.2/2/4 μM | 24 h | DMSO | inhibits TGF-β1–induced Smad2 activation | 22173053 |
| PC-3 | Growth Inhibition Assay | 0.2/2 μM | 24 h | DMSO | blocks the inhibition of cell proliferation produced by TGF-β1 | 22173053 |
| PMOs | Growth Inhibition Assay | 0.2/2 μM | 24 h | DMSO | blocks the inhibition of cell proliferation produced by TGF-β1 | 22173053 |
| U87MG | Growth Inhibition Assay | 5/10 μM | 2 h | enhances radiosensitivity | 22006998 | |
| T98 | Growth Inhibition Assay | 5/10 μM | 2 h | enhances radiosensitivity | 22006998 | |
| U87MG | Apoptosis Assay | 10 μM | 2 h | enhances radiation-induced DNA damage and apoptosis rates | 22006998 | |
| NMA-23 | Apoptosis Assay | 10 μM | 2 h | enhances radiation-induced DNA damage and apoptosis rates | 22006998 | |
| HLE | Function Assay | 0.01-100 nM | 48 h | inhibits the migration in a dose-dependent manner | 20844878 | |
| HLF | Function Assay | 0.01-100 nM | 48 h | inhibits the migration in a dose-dependent manner | 20844878 | |
| 10A/HER2YVMA | Growth Inhibition Assay | 0.1-0.5 μM | 9 d | reduces the size, invasiveness and cell number of colonies | 20383197 | |
| MC38 | Growth Inhibition Assay | 5 μM | 5 d | inhibits cell growth in a time-dependent manner | 19909744 | |
| U937 | Growth Inhibition Assay | 5-20 μM | 24-72 h | inhibits cell growth slightly | 18492113 | |
| HLE | Cytotoxity Assay | 0.001-20 μM | 48 h | induces cell cytotoxity in a dose-dependent manner | 18318443 | |
| HLF | Cytotoxity Assay | 0.001-20 μM | 48 h | induces cell cytotoxity in a dose-dependent manner | 18318443 | |
| Sf9 | Function assay | 1 hr | Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay, IC50 = 0.0037 μM. | 29422332 | ||
| Sf9 | Function assay | Inhibition of TGFbeta type receptor 1 ALK5 (T204D) expressed in Sf9 cells, IC50 = 0.069 μM. | 18314943 | |||
| Mv1Lu-p3TP-Lux | Antiproliferative assay | Antiproliferative activity against mink Mv1Lu-p3TP-Lux cells by luciferase reporter assay, IC50 = 0.18 μM. | 18314943 | |||
| NIH3T3 | Antiproliferative assay | Antiproliferative activity against mouse NIH3T3 cells by [3H]thymidine assay, IC50 = 0.21 μM. | 18314943 | |||
| Calu6 | Function assay | Inhibition of TGFbeta type receptor 1 in orally dosed human Calu6 cells xenografted mouse by IVTI pharmacokinetic assay, EC50 = 0.236 μM. | 18314943 | |||
| T-cells | Function assay | 1 hr | Inhibition of TGFbetaR1 in human primary T-cells assessed as TGFbeta-stimulated PSMAD phosphorylation preincubated for 1 hr followed by TGFbeta addition measured after 90 mins by Alpha screen assay, IC50 = 0.37 μM. | 29422332 | ||
| Mv1Lu | Function assay | 1 hr | Inhibition of TGFbetaR1 in mink Mv1Lu cells assessed as TGFbeta-stimulated PSMAD2 phosphorylation preincubated for 1 hr followed by TGFbeta addition measured after 15 mins, IC50 = 0.75 μM. | 29422332 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 9 mg/mL
(20.38 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 441.52 | Formula | C26H27N5O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 700874-71-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CC2=C(C(=NN2C1)C3=CC=CC=N3)C4=C5C=CC(=CC5=NC=C4)OCCN6CCOCC6 | ||
Mechanism of Action
| Targets/IC50/Ki |
TβRI
(Cell-free assay) 38 nM(Ki)
TβRII
(Cell-free assay) 300 nM(Ki)
|
|---|---|
| In vitro |
LY2109761 treatment induces a dose-dependent low-anchorage growth inhibition of L3.6pl/GLT cells, leading to ~33% or 73% inhibition at 2 μM and 20 μM, respectively, which can be strongly enhanced when in combination index value of 0.36581. Blocking TβRI/II kinase activity with this compound completely suppresses both the basal and TGF-β1-stimulated migration and invasion of L3.6pl/GLT cells, significantly enhances the detachment-induced apoptosis by 26% at 8 hours treatment, and completely suppresses TGF-β–induced Smad2 phosphorylation. This chemical treatment at 1 nM is sufficient to significantly block the migration and invasion but not adhesion of hepatocellular carcinoma cells by increasing E-cadherin expression. It pretreatment enhances radiosensitivity of glioblastoma cells via TGF-β signaling blockage. This compound reduces the self-renewal and proliferation of GBM-derived cancer stem–like cells (CSLC), which can be significantly enhanced when combined with radiation. |
| In vivo |
Administration of LY2109761 (50 mg/kg) alone or in combination significantly reduces the tumor volume by ~70% and ~90%, respectively, prolongs the survival with the median survival duration of 45.0 days and 77.5 days, respectively, and reduces spontaneous abdominal metastases in the L3.6pl/GLT Xenograft mice model. In consistent with the in vitro effect, administration of this compound alone or in combination with radiation, markedly inhibits tumor growth in the orthotopical CSLC glioblastoma model by 43.4% and 76.3%, respectively, decreases tumor invasion and tumor microvessel density, and significantly enhances radiation-induced tumor growth delay in the U87MG xenograft mice model. |
References |
|
Applications
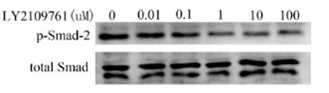
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-Smad2 / Smad E-cadherin β-catenin / MMP-9 / MMP-2 / nm23 / uPA / COX-2 CDK2 / CDK4 / Cyclin D1 / p-Rb |
|
29416682 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































