
- Inhibitors
- By product type
- Natural Products
- Inducing Agents
- Peptides
- Antibiotics
- Antibody-drug Conjugates(ADC)
- PROTAC
- Hydrotropic Agents
- Dyes
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
By research - Antibodies
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- Featured Products
- MRTX1133
- Nab-Paclitaxel
- KP-457
- IAG933
- RMC-6236 (Daraxonrasib)
- RMC-7977
- Zoldonrasib (RMC-9805)
- GsMTx4
- Navitoclax (ABT-263)
- TSA (Trichostatin A)
- Y-27632 Dihydrochloride
- SB431542
- SB202190
- MK-2206 Dihydrochloride
- LY294002
- Alisertib (MLN8237)
- XAV-939
- CHIR-99021 (Laduviglusib)
- Bafilomycin A1 (Baf-A1)
- Thiazovivin (TZV)
- CP-673451
- Verteporfin
- DAPT
- Galunisertib (LY2157299)
- MG132
- SBE-β-CD
- Tween 80
- Bavdegalutamide (ARV-110)
- Z-VAD-FMK
- Wnt-C59 (C59)
- IWR-1-endo
- (+)-JQ1
- 3-Deazaneplanocin A (DZNep) Hydrochloride
- RepSox (E-616452)
- Erastin
- Q-VD-Oph
- Puromycin Dihydrochloride
- Cycloheximide
- Telaglenastat (CB-839)
- A-83-01
- Ceralasertib (AZD6738)
- Liproxstatin-1
- Emricasan (IDN-6556)
- PMA (Phorbol 12-myristate 13-acetate)
- Dibutyryl cAMP (Bucladesine) sodium
- Nedisertib (M3814)
- PLX5622
- IKE (Imidazole Ketone Erastin)
- STM2457
- Saruparib (AZD5305)
- New Products
- Contact Us
research use only
Talazoparib (BMN-673) PARP inhibitor
Cat.No.S7048
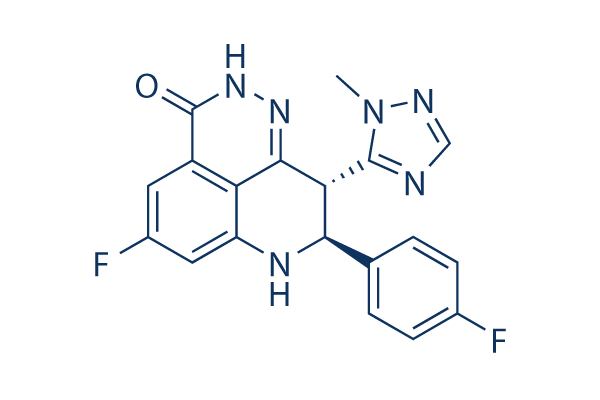
Chemical Structure
Molecular Weight: 380.35
Quality Control
Batch:
Purity:
99.88%
99.88
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| BR5FVB1-Akt | Growth Inhibition Assay | 0.1-100 nM | 24/48/72 h | inhibits cell proliferation dose dependently | 26047697 | |
| BR5FVB1-Akt | Apoptosis Assay | 0.1-100 nM | 72 h | induces apoptosis | 26047697 | |
| Capan-1 | Growth Inhibition Assay | IC50=16.0 ± 5.4 µM | 25864590 | |||
| MIA PaCa-2 | Growth Inhibition Assay | IC50=58.23 ± 8.1 µM | 25864590 | |||
| RD | Growth Inhibition Assay | IC50=8.7 nM | 25263539 | |||
| Rh41 | Growth Inhibition Assay | IC50=8.1 nM | 25263539 | |||
| Rh18 | Growth Inhibition Assay | IC50=4.9 nM | 25263539 | |||
| Rh30 | Growth Inhibition Assay | IC50=31.1 nM | 25263539 | |||
| BT-12 | Growth Inhibition Assay | IC50> 1,000 nM | 25263539 | |||
| CHLA-266 | Growth Inhibition Assay | IC50> 1,000 nM | 25263539 | |||
| TC-71 | Growth Inhibition Assay | IC50=3.7 nM | 25263539 | |||
| CHLA-9 | Growth Inhibition Assay | IC50=8.2 nM | 25263539 | |||
| CHLA-10 | Growth Inhibition Assay | IC50=67.8 nM | 25263539 | |||
| CHLA-258 | Growth Inhibition Assay | IC50=4.6 nM | 25263539 | |||
| SJ-GBM2 | Growth Inhibition Assay | IC50=16.2 nM | 25263539 | |||
| NB-1643 | Growth Inhibition Assay | IC50=18.4 nM | 25263539 | |||
| NB-EBc1 | Growth Inhibition Assay | IC50=25.8 nM | 25263539 | |||
| CHLA-90 | Growth Inhibition Assay | IC50> 1,000 nM | 25263539 | |||
| CHLA-136 | Growth Inhibition Assay | IC50=14.2 nM | 25263539 | |||
| NALM-6 | Growth Inhibition Assay | IC50=49 nM | 25263539 | |||
| COG-LL-317 | Growth Inhibition Assay | IC50=9.4 nM | 25263539 | |||
| RS4;11 | Growth Inhibition Assay | IC50=52.6 nM | 25263539 | |||
| MOLT-4 | Growth Inhibition Assay | IC50=16.6 nM | 25263539 | |||
| CCRF-CEM | Growth Inhibition Assay | IC50=697.3 nM | 25263539 | |||
| Kasumi-1 | Growth Inhibition Assay | IC50=786.2 nM | 25263539 | |||
| Karpas-299 | Growth Inhibition Assay | IC50=75.7 nM | 25263539 | |||
| Ramos-RA1 | Growth Inhibition Assay | IC50=68.3 nM | 25263539 | |||
| DT40 | Growth Inhibition Assay | IC50=4 nM | 24356813 | |||
| DU145 | Growth Inhibition Assay | IC50=11 nM | 24356813 | |||
| H209 | Growth Inhibition Assay | IC50=1.7 nM | 24077350 | |||
| H1048 | Growth Inhibition Assay | IC50=2.2 nM | 24077350 | |||
| H524 | Growth Inhibition Assay | IC50=3.1 nM | 24077350 | |||
| H1930 | Growth Inhibition Assay | IC50=4.1 nM | 24077350 | |||
| H69 | Growth Inhibition Assay | IC50=5.2 nM | 24077350 | |||
| H2081 | Growth Inhibition Assay | IC50=6.3 nM | 24077350 | |||
| H2107 | Growth Inhibition Assay | IC50=7.3 nM | 24077350 | |||
| H1092 | Growth Inhibition Assay | IC50=8.9 nM | 24077350 | |||
| DMS-79 | Growth Inhibition Assay | IC50=9.3 nM | 24077350 | |||
| H446 | Growth Inhibition Assay | IC50=13 nM | 24077350 | |||
| COR-L279 | Growth Inhibition Assay | IC50=15 nM | 24077350 | |||
| LoVo | Function assay | 30 mins | EC50 = 0.0025 μM | 25761096 | ||
| MX1 | Cytotoxicity assay | EC50 = 0.0003 μM | 26652717 | |||
| LoVo | Function assay | 30 mins | EC50 = 0.00251 μM | 26652717 | ||
| LoVo | Cytotoxicity assay | 0.4 uM | 5 days | GI50 = 0.004 μM | 26652717 | |
| Capan1 | Cytotoxicity assay | EC50 = 0.005 μM | 26652717 | |||
| MRC5 | Cytotoxicity assay | EC50 = 0.31 μM | 26652717 | |||
| MX1 | Function assay | 1 mg/kg | 2 ,8 and 24 hrs | Decrease in PAR level in athymic nu/nu mouse xenografted with human MX1 cells at 1 mg/kg, po administered as single dose measured after 2 ,8 and 24 hrs by ELISA | 26652717 | |
| MX1 | Antitumor assay | 0.33 mg/kg | 28 days | Antitumor activity against BRCA1 deficient human MX1 cells xenografted in athymic nu/nu mouse at 0.33 mg/kg, po qd administered for 28 days | 26652717 | |
| MX1 | Antitumor assay | 0.165 mg/kg | 28 days | Antitumor activity against BRCA1 deficient human MX1 cells xenografted in athymic nu/nu mouse assessed as tumor growth inhibition at 0.165 mg/kg, po administered twice a day for 28 days | 26652717 | |
| MX1 | Function assay | 0.33 mg/kg | Potentiation of carboplatin-induced tumor growth inhibition of BRCA1 deficient human MX1 cells xenografted in athymic nu/nu mouse at 0.33 mg/kg po and animals were treated with carboplatin at 35 mg/kg, ip on day 1 | 26652717 | ||
| MDA-MB-436 | Antiproliferative assay | 7 days | IC50 = 0.0007 μM | 28692916 | ||
| Capan1 | Antiproliferative assay | 7 days | IC50 = 0.0018 μM | 28692916 | ||
| VC8 | Cytotoxicity assay | 3 days | IC50 = 0.0042 μM | 28692916 | ||
| V79 | Cytotoxicity assay | 3 days | IC50 = 5.0114 μM | 28692916 | ||
| Capan1 | Function assay | 0.1 uM | 4 hrs | Inhibition of PARP1 in BRCA2 deficient human Capan1 cells assessed as increase in PARP1-DNA trapping at 0.1 uM after 4 hrs by Western blot analysis | 28692916 | |
| MDA-MB-436 | Function assay | 1 uM | 4 hrs | Inhibition of PARP1 in BRCA1 deficient human MDA-MB-436 cells assessed as increase in PARP1-DNA trapping at 1 uM after 4 hrs by Western blot analysis | 28692916 | |
| Click to View More Cell Line Experimental Data | ||||||
Chemical Information, Storage & Stability
| Molecular Weight | 380.35 | Formula |
C19H14F2N6O
|
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1207456-01-6 | -- | Storage of Stock Solutions |
|
|
| Synonyms | LT-673 | Smiles | CN1C(=NC=N1)C2C(NC3=CC(=CC4=C3C2=NNC4=O)F)C5=CC=C(C=C5)F | ||
Solubility
|
In vitro |
DMSO
: 19 mg/mL
(49.95 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Features |
Most potent and selective PARPi reported thus far.
|
|---|---|
| Targets/IC50/Ki |
PARP1 [1]
(Cell-free assay) 0.57 nM
|
| In vitro |
BMN-673 selectively binds to PARP and prevents PARP-mediated DNA repair of single strand DNA breaks via the base-excision repair pathway. This enhances the accumulation of DNA strand breaks, promotes genomic instability and eventually leads to apoptosis. BMN 673 selectively kills cancer cells with BRCA-1 or BRCA-2 mutations. BMN 673 demonstrates single-agent cytotoxicityin BRCA-1 mutant (MX-1, IC50 = 0.3 nM) and BRCA-2 mutant cells (Capan-1, IC50 = 5 nM). In contrast, in MRC-5 normal human fibroblastand other tumor cell lines with wild-type BRCA-1 and BRCA-2 genes, IC50 of BMN 673 ranges between 90 nM and 1.9 μM. [1] Off-target molecular screening did not identify significant non-specific activity for this class of PARP inhibitors. [2] |
| In vivo |
In rat pharmacokinetic studies, BMN 673 displays >50% oralbioavailability and pharmacokinetic properties that enable singledaily dosing. In MX-1 xenograft tumor model studies, daily oral dosingof BMN 673 significantly enhances the antitumor effects ofcytotoxic therapies in a dose-dependent manner. [2] |
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | cleaved-PARP / cleaved-caspase3 / γ-H2AX pKAP1 / pChk2 / pChk1 PD-L1 p-ATM |
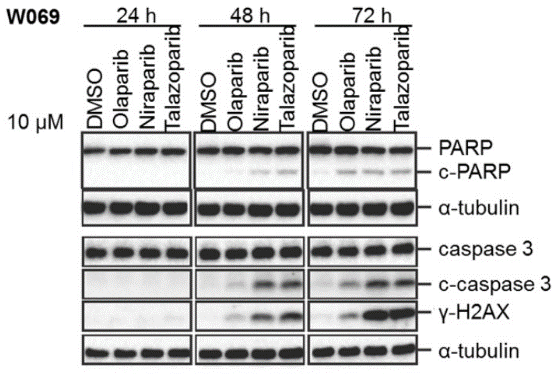
|
29158830 |
| Growth inhibition assay | Cell viability |
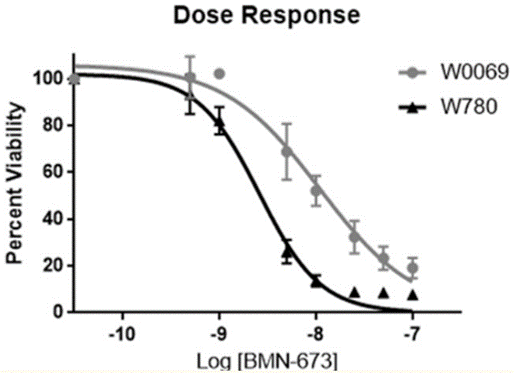
|
29158830 |
| Immunofluorescence | cleaved PARP / 53BP1 RAD51 |
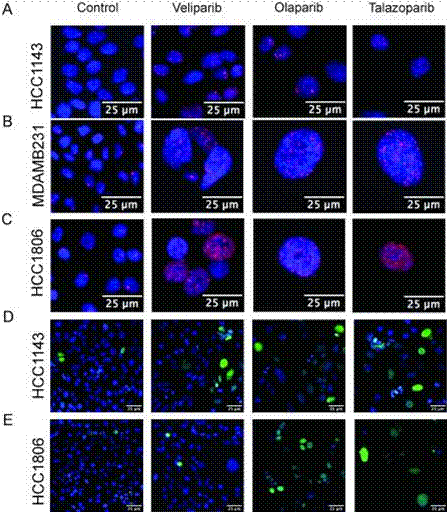
|
28958991 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05425862 | Suspended | Metastatic Castration Resistant Prostate Cancer (mCRPC) |
Peter MacCallum Cancer Centre Australia |
October 21 2022 | Phase 1 |
| NCT05141708 | Completed | Metastatic Breast Cancer|Breast Neoplasms |
Pfizer |
December 17 2021 | -- |
| NCT05053854 | Recruiting | Neuroendocrine Tumors |
Peter MacCallum Cancer Centre Australia |
December 8 2021 | Phase 1 |
| NCT04991480 | Active not recruiting | Advanced Cancer|Metastatic Cancer|Breast Cancer |
Artios Pharma Ltd |
September 13 2021 | Phase 1|Phase 2 |
| NCT04987931 | Completed | Breast Cancer |
Pfizer |
August 20 2021 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
Which solvent do you recommend to dilute it for in vivo study in mice?
Answer:
According to the paper: http://clincancerres.aacrjournals.org/content/19/18/5003.full, it can be dissolved in vehicle (10% DMAc, 6% Solutol, and 84% PBS). Quote from Method and Material section "Xenograft experiments: BMN 673 (various doses as indicated), or vehicle (10% DMAc, 6% Solutol, and 84% PBS) was administered by oral gavage"






































