- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Thiazovivin (TZV) ROCK inhibitor
Cat.No.S1459
Chemical Structure
Molecular Weight: 311.36
Jump to
Quality Control
Batch:
Purity:
99.85%
99.85
Products Often Used Together with Thiazovivin (TZV)
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HSMCs | Function Assay | 1 μM | 2 h | reverses AngII-induced MYPT1 phosphorylation | 24965170 | |
| SCU-i10 hiPSCs | Growth Inhibition Assay | 0-20 μM | 24 h | significantly modulates single-cell plating efficiency and promote NCM growth | 25077932 | |
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 63 mg/mL
(202.33 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 311.36 | Formula | C15H13N5OS |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1226056-71-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1=CC=C(C=C1)CNC(=O)C2=CSC(=N2)NC3=NC=NC=C3 | ||
Mechanism of Action
| Targets/IC50/Ki |
ROCK
(Cell-free assay) ~0.5 μM
|
|---|---|
| In vitro |
Although displaying little impact on cell proliferation, Thiazovivin treatment significantly enhances the survival of human embryonic stem cells (hESCs) after enzymatic dissociation more than 30-fold, while homogenously maintaining pluripotency with the characteristic colony morphology, expression of typical pluripotency markers such as alkaline phosphatase (ALP), and normal karyotype. Dissociated hESCs treated with this compound display dramatically increased adhesion to matrigel- or laminin-coated plates but not to gelatin-coated plates within a few hours. This chemical treatment increases cell-ECM adhesion-mediated β1 integrin activity, which synergizes with growth factors to promote cell survival. In addition to activating integrin, this compound but not Tyrintegin (Ptn) protects hESCs from death in the absence of ECM in suspension through E-cadherin-mediated cell-cell interaction. It potently inhibits endocytosis of E-cadherin, consequently stabilizing E-cadherin on the cell surface and leading to reestablishment of cell-cell interaction, which is essential for hESC survival in ECM-free conditions. This inhibitor but not Tyrintegin (Ptn) at 2 μM inhibits Rho-associated kinase (ROCK) activity and protects hESCs at a similar level as the widely used selective ROCK inhibitor Y-27632 at 10 μM, suggesting that Rho-ROCK signaling regulates cell-ECM and cell-cell adhesion. It at 1 μM increases the reprogramming efficiency of CB mononuclear cells to induced pluripotent stem cells (iPSCs) by more than 10 times. |
| Kinase Assay |
In vitro ROCK assay
|
|
Thiazovivin is dissolved in DMSO. CycLex Rho-kinase assay kit is used to detect ROCK activity using recombinant ROCK in the presence of increasing concentrations of this compound (~10 μM).
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | ROCK1/2 |
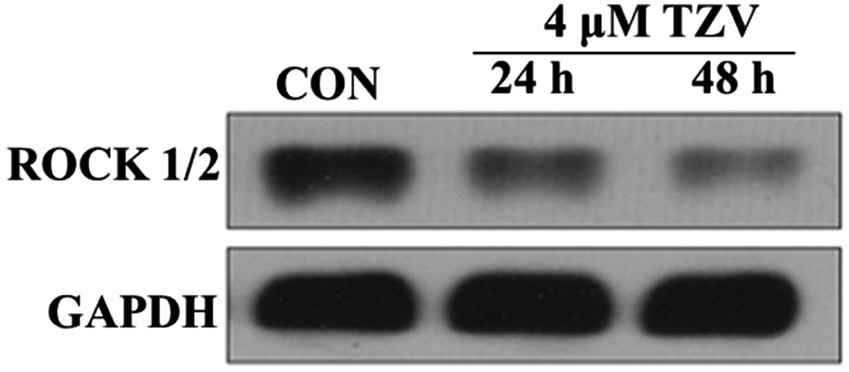
|
28849097 |
| Immunofluorescence | E-cadherin / N-cadherin / α-SMA |
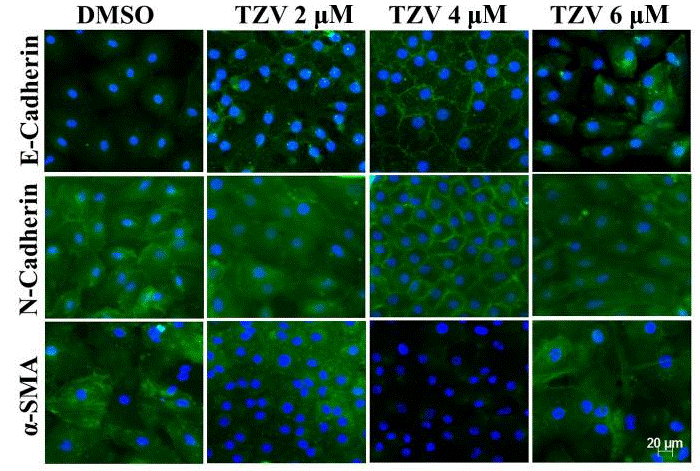
|
28849097 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































