- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Tivozanib
Synonyms: AV-951, KRN-951
Home Protein Tyrosine Kinase VEGFR inhibitor - c-Kit inhibitor - PDGFR inhibitor Tivozanib
For research use only.
Tivozanib is a potent and selective VEGFR inhibitor for VEGFR1/2/3 with IC50 of 30 nM/6.5 nM/15 nM, and also inhibits PDGFR and c-Kit, low activity observed against FGFR-1, Flt3, c-Met, EGFR and IGF-1R. Phase 3.
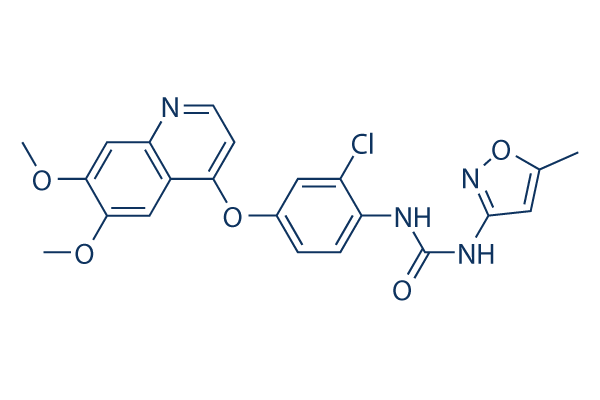
Tivozanib Chemical Structure
CAS No. 475108-18-0
Purity & Quality Control
Batch:
Purity:
99.64%
99.64
Tivozanib Related Products
| Related Targets | VEGFR1 VEGFR2 VEGFR3 | Click to Expand |
|---|---|---|
| Related Products | Cediranib (AZD2171) Vatalanib (PTK787) 2HCl Linifanib (ABT-869) Anlotinib (AL3818) dihydrochloride SAR131675 Apatinib Apatinib (YN968D1) mesylate Ki8751 ZM 323881 HCl Semaxanib (SU5416) SU5402 Cediranib Maleate Motesanib Diphosphate (AMG-706) Brivanib (BMS-540215) ZM 306416 Motesanib (AMG-706) KRN 633 Telatinib Taxifolin (Dihydroquercetin) SKLB1002 | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library Tyrosine Kinase Inhibitor Library PI3K/Akt Inhibitor Library Cell Cycle compound library Angiogenesis Related compound Library | Click to Expand |
Signaling Pathway
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MCF7 cells | Proliferation assay | Antiproliferative activity against human MCF7 cells, IC50=0.38 μM | 24583357 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-SH cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Tivozanib is a potent and selective VEGFR inhibitor for VEGFR1/2/3 with IC50 of 30 nM/6.5 nM/15 nM, and also inhibits PDGFR and c-Kit, low activity observed against FGFR-1, Flt3, c-Met, EGFR and IGF-1R. Phase 3. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
| In vitro | ||||
| In vitro | AV-951 is a novel quinoline-urea derivative. AV-951 blocks VEGF-dependent activation of mitogen-activated protein kinases and proliferation of endothelial cells. [1] | |||
|---|---|---|---|---|
| Kinase Assay | Kinase Assays | |||
| Cell-free kinase assays are done in quadruplicate with 1 μM ATP to determine the IC50 values of AV-951 against a variety of recombinant receptor and nonreceptor tyrosine kinases including VEGFR1, VEGFR2, VEGFR3, c-Kit, PDGFRβ, Flt-3 and FGFR1. | ||||
| Cell Research | Cell lines | Human umbilical vein endothelial cells (HUVEC) and normal human dermal fibroblasts | ||
| Concentrations | 1 μM | |||
| Incubation Time | 15 minutes | |||
| Method | Human umbilical vein endothelial cells (HUVEC) and normal human dermal fibroblasts-based assays are done to determine the ability of AV-951 to inhibit ligand-dependent phosphorylation of tyrosine kinase receptors. The cells are starved overnight in appropriate basic medium containing 0.5% fetal bovine serum (FBS). The cells are incubated for 1 hour following the addition of AV-951 or 0.1% DMSO, and then stimulated with the cognate ligand at 37 °C. Receptor phosphorylation is induced for 5 minutes except for VEGFR3 (10 minutes), c-Met (10 minutes), and c-Kit (15 minutes). All the ligands used in the assays are human recombinant proteins, except for VEGF-C, a rat recombinant protein. Following cell lysis, receptors are immunoprecipitated with appropriate antibodies and subjected to immunoblotting with phosphotyrosine. Quantification of the blots and calculation of IC50 values are carried ou | |||
| In Vivo | ||
| In vivo | In vivo studies show that AV-951 also decreases the micro vessel density and suppresses VEGFR2 phosphorylation levels in tumor xenografts, especially at a concentration of 1mg/kg (p.o. administration). AV-951 shows almost complete inhibition of tumor xenografts growth (TGI>85%) in athymic rats. [1] Another study in rat peritoneal disseminated tumor model shows that AV-951 could prolong the survival of the tumor-bearing rats with the MST of 53.5 days. AV-951 displays antitumor activity against many human tumor xenografts including lung, breast, colon, ovarian, pancreas and prostate cancer. [2] | |
|---|---|---|
| Animal Research | Animal Models | A549 xenografts in Athymic rats (RH-rnu/rnu) |
| Dosages | 1 mg/kg | |
| Administration | Oral administration | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04645160 | Recruiting | Cholangiocarcinoma|Bile Duct Neoplasm|Biliary Tract Malignancy |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
March 4 2022 | Phase 1|Phase 2 |
| NCT03136627 | Completed | Carcinoma Renal Cell |
AVEO Pharmaceuticals Inc.|Bristol-Myers Squibb |
March 22 2017 | Phase 1|Phase 2 |
| NCT01853644 | Completed | Recurrent Epithelial Ovarian Cancer|Recurrent Fallopian Tube Cancer|Recurrent Primary Peritoneal Cancer |
Northwestern University|National Comprehensive Cancer Network |
June 6 2013 | Phase 2 |
| NCT01834183 | Withdrawn | Renal Cell Carcinoma |
Dana-Farber Cancer Institute |
June 2013 | Phase 2 |
| NCT01807156 | Terminated | Hepatocellular Cancer |
Emory University|AVEO Pharmaceuticals Inc. |
March 2013 | Phase 2 |
|
Chemical Information & Solubility
| Molecular Weight | 454.86 | Formula | C22H19ClN4O5 |
| CAS No. | 475108-18-0 | SDF | Download Tivozanib SDF |
| Smiles | CC1=CC(=NO1)NC(=O)NC2=C(C=C(C=C2)OC3=C4C=C(C(=CC4=NC=C3)OC)OC)Cl | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 20 mg/mL ( (43.96 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Frequently Asked Questions
Question 1:
Would you please provide a little bit more detail regarding how to prepare the Tivozanib for in vivo treatment and the storage condition?
Answer:
For in vivo formula, we recommend to use 2% DMSO+30% PEG 300+ddH2O up to 1mg/mL. Once dissolved it in solution, please make small aliquots and store them at -80C up to 6 months without repeated thawing and refreezing.