- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
Apatinib (YN968D1) mesylate VEGFR inhibitor
Apatinib mesylate (YN968D1, Rivoceranib) is a potent inhibitor of the VEGF signaling pathway with IC50 values of 1 nM, 13 nM, 429 nM and 530 nM for VEGFR-2, Ret (c-Ret), c-Kit and c-Src, respectively. Apatinib mesylate induces both autophagy and apoptosis.
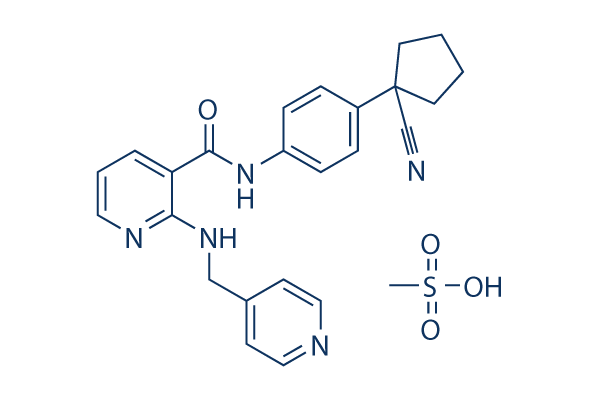
Chemical Structure
Molecular Weight: 493.58
Purity & Quality Control
Batch:
Purity:
99.77%
99.77
Related Products
| Related Targets | VEGFR1 VEGFR2 VEGFR3 | Click to Expand |
|---|---|---|
| Related Products | Cediranib (AZD2171) Vatalanib (PTK787) 2HCl Linifanib (ABT-869) Anlotinib (AL3818) dihydrochloride SAR131675 Apatinib Ki8751 ZM 323881 HCl Semaxanib (SU5416) SU5402 Cediranib Maleate Motesanib Diphosphate (AMG-706) Brivanib (BMS-540215) Motesanib (AMG-706) ZM 306416 KRN 633 Telatinib Fruquintinib SKLB1002 Taxifolin (Dihydroquercetin) | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library Tyrosine Kinase Inhibitor Library PI3K/Akt Inhibitor Library Cell Cycle compound library Angiogenesis Related compound Library | Click to Expand |
Signaling Pathway
Mechanism of Action
| Features | Good anti-tumor effects for gastric and colorectal cancer compared with NSC-724772 and sunitinib. | ||||
|---|---|---|---|---|---|
| Targets |
|
In vitro |
||||
| In vitro | Apatinib (YN968D1) is a novel, orally bioavailable, selective inhibitor with potential antiangiogenic and antineoplastic activities. Apatinib selectively binds to and inhibits VEGFR2. Apatinib can also potently suppress the activities of Ret, c-kit and c-src with IC50 of 0.013 μM, 0.429 μM and 0.53 μM, respectively. Apatinib inhibits cellular phosphorylation of VEGFR-2, c-kit and PDGFRβ. Apatinib significantly inhibits proliferation stimulated by 20 ng/mL VEGF (IC50 = 0.17μM). Apatinib effectively inhibits proliferation, migration and tube formation of human umbilical vein endothelial cells induced by FBS, and blocked the budding of rat aortic ring. [1] Apatinib reverses ABCB1- and ABCG2-mediated MDR by inhibiting their transport function, but not by blocking the AKT or ERK1/2 pathway or downregulating ABCB1 or ABCG2 expression. Apatinib significantly potentiates the cytotoxicity of established ABCB1 and ABCG2 substrates and increased the accumulation of DOX and Rho 123 in ABCB1- or ABCG2-overexpressing cells. Furthermore, apatinib significantly inhibited the photoaffinity labeling of both ABCB1 and ABCG2 with [125I]iodoarylazidoprazosin in a concentration-dependent manner. [2] |
|||
|---|---|---|---|---|
| Kinase Assay | Enzyme-linked immunosorbent assay | |||
| A poly(glu, ala, tyr) 6:3:1 random copolymer is used as a tyrosine containing substrate solution. The substrate is stored as a 1 mg/mL stock in PBS at −20 °C and diluted 1 in 500 with PBS in order to coat 96 well plates (100 μL/well). Plates are coated on the day prior to assay, sealed with adhesive seals, and stored overnight at 4 °C. On the day of the assay, the substrate solution is discarded and the assay plate wells are washed once with PBST (PBS containing 0.05% v/v Tween 20) and once with Hepes buffer (50 mM, pH 7.4).Test compounds are diluted with 10% DMSO de-ionized water and 25 μL volumes transferred to wells in the washed assay plates. Manganese chloride solution (40 mM) containing 8 μM ATP is then added (25 μL) to all test wells. Control and blank wells, containing compound diluent and manganese chloride solution with and without ATP, respectively, are also included to determine the dynamic range of the assay. Freshly diluted enzyme (50 μL) is added to each well, and the plates incubated at room temperature for 20 min. The liquid is then discarded and the wells are washed twice with PBST. Mouse IgG anti-phosphotyrosine antibody diluted 1:6000 with PBST containing 0.5% (w/v) bovine serum albumin (BSA) is added (100 μL/well), and the plates incubated for 1h at room temperature before discarding the liquid and washing the wells twice with PBST. Horseradish peroxidase (HRP)-linked sheep anti-mouse Ig antibody diluted 1:500 with PBST containing 0.5% (w/v) BSA, is then added (100 μL/well) and the plates incubated for a further 1 h at room temperature before discarding the liquid and washing the wells twice with PBST. A 1 mg/mL solution of 2,2‘-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid is freshly prepared in 50 mM phosphate-citrate buffer (pH5.0) containing 0.03% (w/v) sodium perborate, and 100 μL added to each well. Plates are then incubated for 20−60 min at room temperature until the optical density value of control wells measured at 405 nm is approximately 1.0. IC50 values for compound enzyme inhibition are interpolated using Microcal Origin following subtraction of blank values. | ||||
| Cell Research | Cell lines | HUVEC | ||
| Concentrations | ~25 μM | |||
| Incubation Time | 72 h | |||
| Method | The HUVEC are seeded into 96-well plates. After 24 h of incubation, cells are exposed to the test agents (vehicle as control) together with 20 ng/mL VEGF or 20% FBS for another 72 h. After fixation with 10% trichloroacetic acid, the cells are stained with 0.4% sulforhodamine B for 30 min at 37 °C and then washed with 1% acetic acid. Tris is added to dissolve the complex, and the optical density is measured at 520 n |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p-VEGFR2 / VEGFR2 / p-ERK / ERK PI3K / p-PI3K / mTOR / p-mTOR / AKT / p-AKT Beclin 1 / Atg7 / p62 / LC3-I / LC3-II |
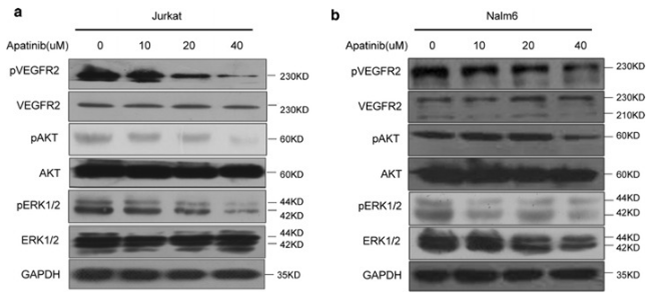
|
29490645 | |
| Growth inhibition assay | Cell viability |
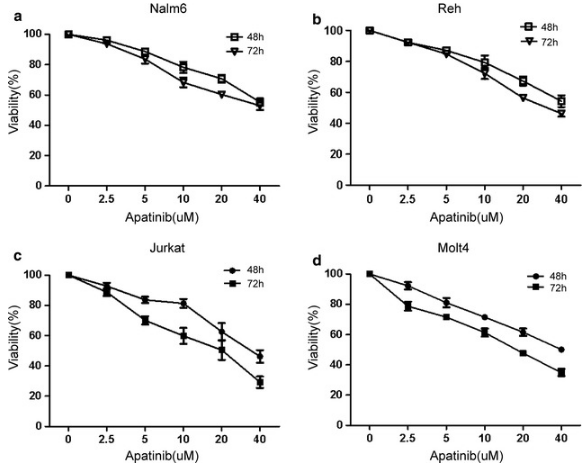
|
29490645 | |
In Vivo |
||
| In vivo | Apatinib inhibits the growth of a broad range of human tumor xenografts in a significant dose-dependent manner. [1] Apatinib reverses ABCB1-mediated MDR in the nude mouse xenograft model. [2] Apatinib significantly enhances the antitumor activity of doxorubicin in nude mice bearing K562/ADR xenografts. [3] |
|
|---|---|---|
| Animal Research | Animal Models | Ls174t, HCT 116, SGC-7901, HT-29, A549, NCI-H460 xenografted BALB/cA nude mice |
| Dosages | 50, 100, 200 mg/kg | |
| Administration | p.o. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05839197 | Recruiting | Macrotrabecular Massive Hepatocellular Carcinoma |
Wan-Guang Zhang|Tongji Hospital |
May 5 2023 | Phase 2 |
| NCT05742750 | Not yet recruiting | Locally Advanced Biliary Tract Cancer|Metastatic Biliary Tract Cancer |
Sun Yat-sen University|Jiangsu Hengrui Pharmaceutical Co. Ltd. |
March 1 2023 | Phase 1|Phase 2 |
| NCT05287360 | Completed | Healthy |
Elevar Therapeutics |
December 30 2021 | Phase 1 |
| NCT03743428 | Suspended | Colorectal Neoplasms |
Shenzhen People''s Hospital |
October 22 2020 | Not Applicable |
| NCT04517357 | Unknown status | Relapsed Ovarian Cancer |
Jiangsu HengRui Medicine Co. Ltd. |
October 16 2020 | Phase 2 |
References |
|
Chemical Information
| Molecular Weight | 493.58 | Formula | C25H27N5O4S |
| CAS No. | 1218779-75-9 | SDF | Download SDF |
| Synonyms | YN968D1, Rivoceranib | ||
| Smiles | C[S](O)(=O)=O.O=C(NC1=CC=C(C=C1)C2(CCCC2)C#N)C3=CC=CN=C3NCC4=CC=NC=C4 | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 99 mg/mL ( (200.57 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Frequently Asked Questions
Question 1:
How to reconstitute the compound S2221 for in vivo studies?
Answer:
We suggest the vehicle 0.5% CMC. In vehicle 0.5% CMC, the compound is not fully dissolved. However, the mixture is a stable suspension and can be used for oral gavage feeding.