- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
GSK343 Histone Methyltransferase inhibitor
GSK343 is a potent and selective EZH2 inhibitor with IC50 of 4 nM in a cell-free assay, showing 60 fold selectivity against EZH1, and >1000 fold selectivity against other histone methyltransferases. This compound induces autophagy.
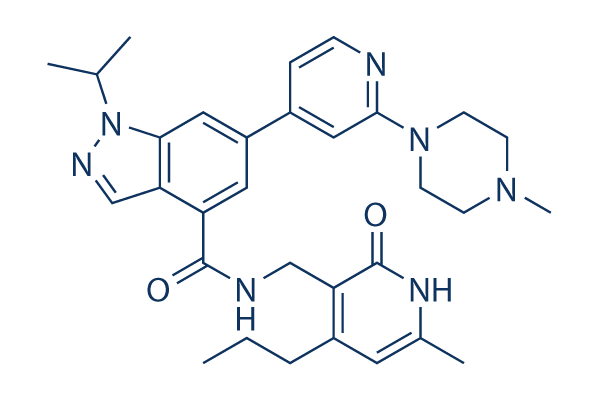
Chemical Structure
Molecular Weight: 541.69
Purity & Quality Control
Batch:
Purity:
99.95%
99.95
Related Products
| Related Targets | Menin-MLL interaction WDR5-MLL interaction | Click to Expand |
|---|---|---|
| Related Products | GSK126 3-Deazaneplanocin A (DZNep) Hydrochloride Pinometostat (EPZ5676) BIX-01294 trihydrochloride EPZ004777 EPZ015666 (GSK3235025) UNC1999 Chaetocin EPZ005687 Pemrametostat (GSK3326595) SGC 0946 MM-102 UNC0638 EPZ011989 UNC0642 OICR-9429 UNC0379 GSK591 Lirametostat (CPI-1205) MI-2 (Menin-MLL Inhibitor) | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library FDA-approved Drug Library Natural Product Library Bioactive Compound Library-I Highly Selective Inhibitor Library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| 2D10 | Function Assay | 0.25-2.0 μM | 96 h | reduces total cellular trimethylated H3K27 in a time- and dose-dependent manner | 26041287 | |
| MDA-MB-231 | Growth Inhibition Assay | 0-20 μM | 72 h | shows cytotoxicity in a dose-dependent manner | 25203626 | |
| MDA-MB-231 | Function Assay | 10 μM | 72 h | reduces the level of H3K27-me3 | 25203626 | |
| MDA-MB-231 | Function Assay | 10 μM | 72 h | induces LC3-II accumulation | 25203626 | |
| MDA-MB-231 | Function Assay | 10 μM | 24 h | induces autophagy | 25203626 | |
| HepG2 | Growth Inhibition Assay | 0-20 μM | 72 h | shows cytotoxicity in a dose-dependent manner | 25203626 | |
| A549 | Growth Inhibition Assay | 0-20 μM | 72 h | shows cytotoxicity in a dose-dependent manner | 25203626 | |
| HepG2 | Function Assay | 10 μM | 24 h | induces autophagy | 25203626 | |
| A549 | Function Assay | 10 μM | 24 h | induces autophagy | 25203626 | |
| OVCAR10 | Growth Inhibition Assay | 1 μM | 0-12 d | DMSO | inhibits cell growth time dependently | 23759589 |
| UPN289 | Growth Inhibition Assay | 1 μM | 0-12 d | DMSO | inhibits cell growth time dependently | 23759589 |
| SKOV3 | Growth Inhibition Assay | 1 μM | 0-12 d | DMSO | inhibits cell growth time dependently | 23759589 |
| SKOV3 | Apoptosis Assay | 1 μM | 4 d | DMSO | induces apoptosis cultured in 3D conditions | 23759589 |
| HCC1806 | Function assay | 72 hrs | Inhibition of EZH2-mediated nuclear H3K27 methylation in human HCC1806 cells after 72 hrs by immunofluorescence analysis, IC50 = 0.174 μM. | 24900432 | ||
| LNCaP | Function assay | 6 days | Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis, IC50 = 2.9 μM. | 24900432 | ||
| DU145 | Function assay | 6 days | Inhibition of EZH2-mediated proliferation of human DU145 cells after 6 days by chemiluminescence analysis | 24900432 | ||
| SKBR3 | Function assay | 6 days | Inhibition of EZH2-mediated proliferation of human SKBR3 cells after 6 days by chemiluminescence analysis | 24900432 | ||
| PC3 | Function assay | 6 days | Inhibition of EZH2-mediated proliferation of human PC3 cells after 6 days by chemiluminescence analysis | 24900432 | ||
| ZR-75-1 | Function assay | 6 days | Inhibition of EZH2-mediated proliferation of human ZR-75-1 cells after 6 days by chemiluminescence analysis | 24900432 | ||
| HCC180 | Function assay | 6 days | Inhibition of EZH2-mediated proliferation of human HCC180 cells after 6 days by chemiluminescence analysis | 24900432 | ||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Features | A chemical probe for the SGC epigenetics initiative. Potential use in a variety of solid tumors. | ||||
|---|---|---|---|---|---|
| Targets |
|
In vitro |
||||
| In vitro | GSK343 inhibits trimethylation of H3K27 (H3K27me3) with IC50 of 174 nM in HCC1806 breast cancer cells. This compound potently inhibits cell proliferation in breast cancer cells and prostate cancer cells, and the prostate cancer cell line LNCaP is the most sensitive to it, with IC50 of 2.9 μM. [1] This chemical significantly suppresses the growth of EOC cells cultured in 3D matrigel extracellular matrix (ECM), which mimics the tumor microenvironment in vivo. In addition, it also induces apoptosis of EOC cells in 3D and significantly inhibits the invasion of EOC cells. [2] |
|||
|---|---|---|---|---|
| Kinase Assay | In vitro biochemical assays against histone methyltransferases | |||
| Activity against EZH2 is assessed using 5 member PRC2 complex (Flag-EZH2, EED, SUZ12, AEBP2, RbAp48). The assay protocol may be summarized as follows: 10 mM stocks of compounds are prepared from solid in 100% DMSO. An 11 point serial dilution master plate is prepared in 384 well format (1:3 dilution, columns 6 and 18 were equal volume DMSO controls) and dispensed to assay ready plates using acoustic dispensing technology to create a 100 nL stamp of compound and DMSO controls. The assay additions consisted of equal volume additions of 10 nM EZH2 and the substrate solution (5 µg/mL HeLa nucleosomes and 0.25 µM [3H]-SAM) dispensed into assay plates using a multi-drop combi dispense. Reaction plates are incubated for 1 hr and quenched with an equal volume addition of 0.5 mg/mL PS-PEI Imaging Beads (RPNQ0098) containing 0.1 mM unlabeled SAM. The plates are sealed, dark adapted for 30 minutes, and a 5 minute endpoint luminescence image is acquired using a Viewlux imager. Plate statistics such as Z’ and signal to background as well as dose response curves are analyzed using Activity BaseXE. The in vitro biochemical activity of EZH1 is assessed as part of a 5 member PRC2 complex using a 384 well SPA assay identical to EZH2. Buffer components, reagent dispensing, compound plate preparation, quench conditions and data analysis are identical for EZH1 and EZH2 with final assay concentrations of 20 nM EZH1, 5 μM/mL HeLa nucleosomes and 0.25 μM [3H]-SAM. Further data analysis, pIC50 pivots and visualizations are enabled by TIBCO Spotfire. This compound is profiled at Reaction Biology Corp. (Malvern, PA) to assess inhibition in their panel of histone methyltransferase assays. Methyltransferase activity is assessed using HotSpot technology, a miniaturized radioisotope-based filter binding assay. Inhibitors are dissolved in dimethyl sulfoxide (DMSO) and tested at concentrations up to 100 uM with a final DMSO concentration of 2%. Buffer containing the methyltrasferase at the listed concentration and its preferred substrate as shown in the accompanying table is preincubated with this chemical for 10 min. Reactions are initiated by the addition of 1 uM S-adenosyl-L-[methyl-3H]methionine (SAM), allowed to incubate for 60 min at 30C followed by transfer to P81 filter-paper and PBS wash before detection. | ||||
| Cell Research | Cell lines | Breast cancer cell lines (HCC1806, Sk-Br-3, ZR-75-1), prostate cancer cell lines (DU145, PC3, LNCaP) | ||
| Concentrations | ~50 μM | |||
| Incubation Time | 6 days | |||
| Method | To account for varying doubling rates among cancer cell lines, the optimal cell seeding is determined empirically for all cell lines by examining their growth in a 384-well plate over 6 days with a wide range of seeding densities. Cells are then plated at the optimal seeding density and allowed to adhere overnight. Cells are treated in duplicate with a 20-point 2-fold dilution series of this compound or 0.147% DMSO (vehicle control) and incubated for 6 days at 37C in 5% CO2. Cells are then lysed with 25 μl CellTiter-Glo per well and chemiluminescence is quantified with a TECAN Safire2 microplate reader. In addition, an untreated plate of cells is harvested at the time of compound addition (T0) to quantify the starting number of cells. CTG values after 6 days of treatment were expressed as a percent of the T0 value and plotted against compound concentration. Data are fit with a 4-parameter equation to generate a concentration response curve and the concentration of compound required to inhibit 50% of growth (gIC50) is determined |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | H3K27me3 EZH2 / EED / SUZ12 N-cadherin / Vimentin / Snail / Slug / MMP2 / MMP9 |
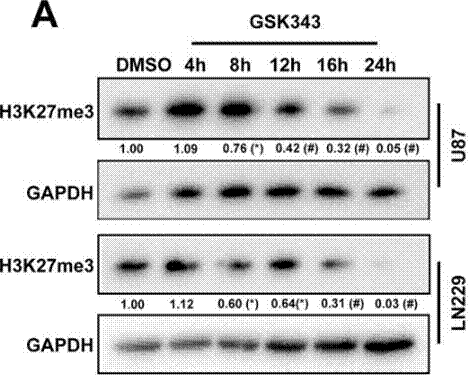
|
29228694 | |
| Immunofluorescence | N-cadherin / H3K27me3 / Vimentin |
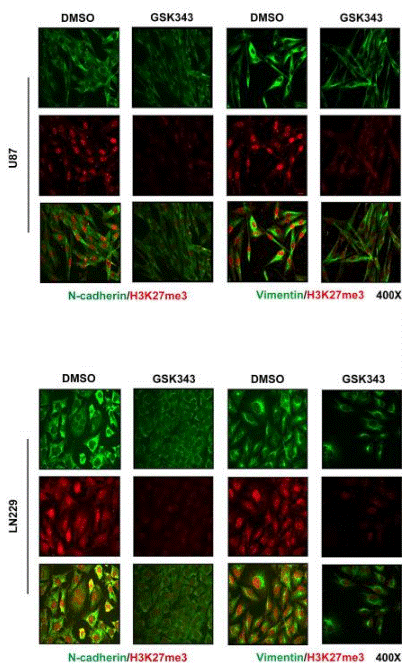
|
29228694 | |
| Growth inhibition assay | Cell viability |
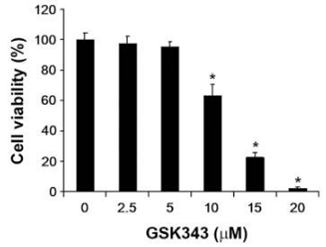
|
26973856 | |
In Vivo |
||
| In vivo | GSK343, a selective EZH2 inhibitor, inhibits phagocytosis. |
|
|---|---|---|
References |
|
Chemical Information
| Molecular Weight | 541.69 | Formula | C31H39N7O2 |
| CAS No. | 1346704-33-3 | SDF | Download SDF |
| Synonyms | N/A | ||
| Smiles | CCCC1=C(C(=O)NC(=C1)C)CNC(=O)C2=C3C=NN(C3=CC(=C2)C4=CC(=NC=C4)N5CCN(CC5)C)C(C)C | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
Ethanol : 4 mg/mL DMSO : 1 mg/mL ( (1.84 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field