- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
U0126-EtOH
Home MAPK MEK inhibitor - Autophagy inhibitor - Antiviral inhibitor - Mitophagy inhibitor U0126-EtOH
For research use only.
U0126-EtOH is a highly selective inhibitor of MEK1/2 with IC50 of 0.07 μM/0.06 μM in cell-free assays, 100-fold higher affinity for ΔN3-S218E/S222D MEK than PD98059. U0126 inhibits autophagy and mitophagy with antiviral activity.
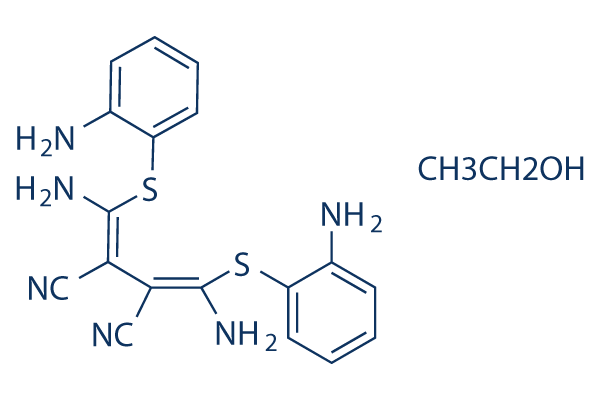
U0126-EtOH Chemical Structure
CAS No. 1173097-76-1
Purity & Quality Control
Batch:
Purity:
99.98%
99.98
Products often used together with U0126-EtOH
U0126-EtOH and SP600125 reverse several genes upregulation in RAW264.7 and Ana-1 cells caused by Echinococcus multilocularis soluble antigen.
U0126-EtOH and Adezmapimod ameliorate indoxyl sulfate-induced inhibitory effects and reductions in Kv4.2, Kv4.3, and KChIP2 proteins and genes.
U0126-EtOH in the presence of Purmorphamine inhibits sonic hedgehog-induced levels of p-ERK1/2 in RA-FLSs.
U0126-EtOH Related Products
| Related Targets | MEK1 MEK1/2 MEK2 MEK5 | Click to Expand |
|---|---|---|
| Related Products | Mirdametinib (PD0325901) PD98059 PD184352 (CI-1040) BIX 02189 Pimasertib (AS-703026) Refametinib (RDEA119) TAK-733 AZD8330 BIX 02188 GDC-0623 SL-327 Myricetin BI-847325 PD318088 APS-2-79 HCl | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library MAPK Inhibitor Library Cell Cycle compound library TGF-beta/Smad compound library Anti-alzheimer Disease Compound Library | Click to Expand |
Signaling Pathway
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| rat PC12 cells | Function assay | 10 μM | 1 h | Activation of Nrf2/ARE in rat PC12 cells assessed as HO-1 protein induction at 10 uM after 5 hrs pretreated with JNK inhibitor SP600125 for 1 hr before compound addition by Western blot analysis | 21345685 | |
| mouse RAS-3T3 cells | Function assay | 10-40 μM | Inhibition of MEK-mediated ERK1/2 phosphorylation in mouse RAS-3T3 cells at 10 to 40 uM by ELISA. | 24507826 | ||
| HCT116 cells | Function assay | Ability of compound to inhibit anchorage independent colony formation (soft agar growth assay) in HCT116 cells, IC50=19.4 μM. | 15225706 | |||
| COS-7 cells | Function assay | Inhibitory concentration against AP-1 transcription in COS-7 cells, IC50=1 μM. | 15006386 | |||
| HeLa cells | Function assay | Inhibition of EGF-stimulated Elk1-luciferase reporter assay in HeLa cells, IC50=0.29 μM. | 15225706 | |||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | U0126-EtOH is a highly selective inhibitor of MEK1/2 with IC50 of 0.07 μM/0.06 μM in cell-free assays, 100-fold higher affinity for ΔN3-S218E/S222D MEK than PD98059. U0126 inhibits autophagy and mitophagy with antiviral activity. | ||||
|---|---|---|---|---|---|
| Features | A chemically synthesized and highly selective inhibitor of both MEK1 and MEK2. | ||||
| Targets |
|
| In vitro | ||||
| In vitro | U0126-EtOH functionally antagonizes AP- 1 transcriptional activity and blocks the production of a variety of cytokines and metalloproteinases involved in the inflammatory response. [1] U0126-EtOH inhibits T cell proliferation in response to antigenic stimulation or cross-linked anti-CD3 plus anti-CD28 Abs without effect on IL-2-induced proliferation by down-regulating IL-2 mRNA levels. [2] A recent study shows that U0126-EtOH antagonizes resveratrol-induced apoptosis in castration-resistant human prostate cancer C4-2 cells, inhibits mitochondrial function and shifts cells to aerobic glycolysis independently of MEK. [3] |
|||
|---|---|---|---|---|
| Kinase Assay | In Vitro Kinase Assays | |||
| The amount of immunoprecipitated wild type MEK used in these assays is adjusted to give a similar amount of activity units as obtained with 10 nM recombinant MEK. Reaction velocities are measured using a 96-well nitrocellulose filter apparatus as described below. Unless otherwise noted, reactions are carried out at an enzyme concentration of 10 nM, in 20 mM Hepes, 10 mM MgCl2, 5 mM β-mercaptoethanol, 0.1 mg/mL BSA, pH 7.4, at room temperature. Reactions are initiated by the addition of [γ-33P]ATP into the premixed MEK/ERK/inhibitor reaction mixture, and an aliquot of 100 μL is taken every 6 minutes and transferred to the 96-well nitrocellulose membrane plate which has 50 mM EDTA to stop the reaction. The membrane plate is drawn and washed 4 times with buffer under vacuum. Wells are then filled with 30 μL of Microscint-20 scintillation fluid, and the radioactivity of 33P-phosphorylated ERK is counted with a Top Count scintillation counter. Velocities are obtained from the slopes of radioactivity versus time plots. Concentrations of ERK and ATP are 400 nM and 40 μM, respectively, unless otherwise indicated. For all of the in vitro enzyme assays, the percent inhibition is calculated 100 (1 −Vi/Vo) where Vi and Vo are the initial reaction velocities in the presence and absence of inhibitor, respectively. The data are then plotted as percent inhibition as a function of inhibitor concentration and fit, by nonlinear least squares regression, to the standard equation for a Langmuir isotherm to determine the IC50. As reported, enzyme concentrations are based upon molecular weights and mass of protein used in the final assay volume and not on active site titration. Thus, the actual enzyme active site concentration may differ from that reported. | ||||
| Cell Research | Cell lines | A.E7 or Th17 cells | ||
| Concentrations | 0 to 10 μM | |||
| Incubation Time | 48 hours | |||
| Method | A.E7 or Th17 cells are incubated with C-treated B10.BR or BALB/c splenocytes plus varying concentrations of pigeon cytochrome c or PR8 Ag, or with 5 U/mL human rIL-2. In addition, some assays contains U0126 or an inactive analogue, U0124, to determine direct effects of MEK inhibition on T cell proliferation. Two days after culture initiation, each well is pulsed with 1 µCi of [3H]TdR and harvested the following day. The incorporation of [3H]TdR into DNA is quantitated on a Packard Matrix 96 direct beta counter without the use of liquid scintillation mixtures. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p-MEK / MEK / p-ERK / ERK E-cadherin / Vimentin / Twist-2 |
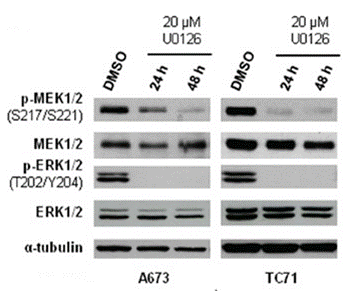
|
27487136 | |
| Immunofluorescence | pERK / pPPARγ E-cadherin / Vimentin CD40 |
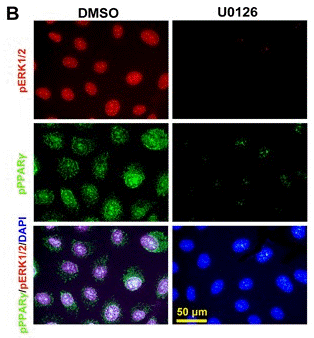
|
27145370 | |
| In Vivo | ||
| In vivo | U0126-EtOH, as the inhibitor of intracellular Raf/MEK/ERK signaling pathway, demonstrates antiviral activity by suppressing propagation of the 2009 pandemic IV H1N1 variant and highly pathogenic avian influenza viruses (HPAIV) in vivo in the mouse lung by inhibiting. [4] U0126-EtOH shows the potential neuroprotective effect and improving spatial learning in Morris water maze (MWM) by activating peroxisome proliferator-activated receptor gamma coactivator-1a, nuclear respiratory factor 1, and mitochondrial transcription factor A in Aβ-injected rats. [5] |
|
|---|---|---|
| Animal Research | Animal Models | Female C57Bl/6 mice infected by Mouse-adapted highly pathogenic avian influenza A/FPV/Bratislava/79 (H7N7; FPV) virus and swine origin human influenza A virus (SOIV) A/Regensburg/D6/2009 (H1N1v; RB1). |
| Dosages | ≤10 mM | |
| Administration | Administered via aerosol. | |
|
Chemical Information & Solubility
| Molecular Weight | 426.56 | Formula | C18H16N6S2.C2H6O |
| CAS No. | 1173097-76-1 | SDF | Download U0126-EtOH SDF |
| Smiles | CCO.C1=CC=C(C(=C1)N)SC(=C(C#N)C(=C(N)SC2=CC=CC=C2N)C#N)N | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 85 mg/mL ( (199.26 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Frequently Asked Questions
Question 1:
I want to know whether the compound is light-sensitive?
Answer:
S1102 U0126-EtOH is not stable. It should be stored as powder at -20°C, and prepared the solution just before use.