research use only
Ruxolitinib (INCB18424) JAK1/2 inhibitor
Cat.No.S1378
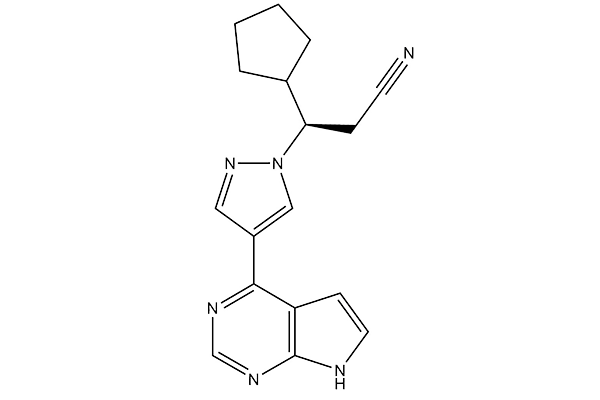
Chemical Structure
Molecular Weight: 306.37
Quality Control
Products Often Used Together with Ruxolitinib (INCB18424)
| Related Targets | EGFR STAT Pim |
|---|---|
| Other JAK Inhibitors | AZD1480 WP1066 Momelotinib (CYT387) Filgotinib (GLPG0634) AT9283 Gandotinib (LY2784544) TG101209 Cerdulatinib (PRT062070) hydrochloride Pacritinib NVP-BSK805 2HCl |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SNU423 | Function Assay | 50 μM | 24 h | DMSO | Inhibition of STAT1 and STAT3 phosphorylation significantly | 23941832 |
| SNU182 | Function Assay | 50 μM | 24 h | DMSO | Inhibition of STAT1 and STAT3 phosphorylation significantly | 23941832 |
| HuH7 | Function Assay | 50 μM | 24 h | DMSO | Inhibition of STAT1 and STAT3 phosphorylation significantly | 23941832 |
| SNU423 | Growth Inhibition Assay | 50 μM | 48 h | DMSO | >81% reduction | 23941832 |
| SNU182 | Growth Inhibition Assay | 50 μM | 48 h | DMSO | >64% reduction | 23941832 |
| HuH7 | Growth Inhibition Assay | 50 μM | 48 h | DMSO | >82% reduction | 23941832 |
| RKO | Apoptosis Assay | 25 μM | 48 h | DMSO | Induces apoptosis by activating caspase 3 | 24050550 |
| DLD-1 | Apoptosis Assay | 25 μM | 48 h | DMSO | Induces apoptosis by activating caspase 3 | 24050550 |
| DLD-1 | Growth Inhibition Assay | 50 μM | 48 h | DMSO | IC50=15.51 μM | 24050550 |
| RKO | Growth Inhibition Assay | 50 μM | 48 h | DMSO | IC50=14.76 μM | 24050550 |
| RKO | Kinase Assay | 25 μM | 48 h | DMSO | does not inhibit JAK1 phosphorylation | 24050550 |
| DLD-1 | Kinase Assay | 25 μM | 48 h | DMSO | Inhibition of JAK2 phosphorylation | 24050550 |
| RKO | Kinase Assay | 25 μM | 48 h | DMSO | Inhibition of JAK1 phosphorylation | 24050550 |
| DLD-1 | Kinase Assay | 25 μM | 48 h | DMSO | Inhibition of JAK1 phosphorylation | 24050550 |
| BaF3 | Kinase Assay | 80 nM | 6 h | DMSO | Reduces the phosphorylation of STAT5 in JAK2V617F-mutated BAF3-EPOR cell | 24237791 |
| Huh7 | Function Assay | 1 μM | 16 h | DMSO | Impaires the capacity of IHCA-associated gp130 mutants to signal to STAT3 | 24501689 |
| HepG2 | Function Assay | 1 μM | 16 h | DMSO | Impaires the capacity of IHCA-associated gp130 mutants to signal to STAT3 | 24501689 |
| Hep3B | Function Assay | 1 μM | 16 h | DMSO | Impaires the capacity of IHCA-associated gp130 mutants to active STAT3 with IC50 of ~50 μM | 24501689 |
| NCI-H2347 | Apoptosis Assay | 30 nM | 48 h | DMSO | Induction of apoptosis | 25213670 |
| NCI-H1299 | Apoptosis Assay | 30 nM | 48 h | DMSO | Induction of apoptosis | 25213670 |
| A549/DDP | Apoptosis Assay | 30 nM | 48 h | DMSO | Induction of apoptosis | 25213670 |
| NCI-H1299 | Function Assay | 30 nM | 48 h | DMSO | Down-regulation of STAT3 phosphorylation | 25213670 |
| NCI-H2347 | Function Assay | 30 nM | 48 h | DMSO | Decrease in Bcl2 expression | 25213670 |
| A549/DDP | Function Assay | 30 nM | 48 h | DMSO | Down-regulation of STAT3 phosphorylation | 25213670 |
| NCI-H2347 | Growth Inhibition Assay | DMSO | IC50=0.17 μM | 25213670 | ||
| NCI-H1299 | Growth Inhibition Assay | DMSO | IC50=0.28 μM | 25213670 | ||
| A549/DDP | Growth Inhibition Assay | DMSO | IC50=0.22 μM | 25213670 | ||
| A549 | Growth Inhibition Assay | DMSO | IC50=0.04 μM | 25213670 | ||
| NCI-H358 | Growth Inhibition Assay | DMSO | IC50=0.1 μM | 25213670 | ||
| NCI-H460 | Growth Inhibition Assay | DMSO | IC50=0.13 μM | 25213670 | ||
| CMK | Growth Inhibition Assay | Inhibition of CMK carrying the WT JAK cell proliferation with IC50 of 0.075 μM | 25352124 | |||
| CMK | Growth Inhibition Assay | Inhibition of CMK carrying the JAK3A63D mutation cell proliferation with IC50 of 0.163 μM | 25352124 | |||
| CMK | Growth Inhibition Assay | Inhibition of CMK carrying the JAK3A572V mutation cell proliferation | 25352124 | |||
| HT93A | Growth Inhibition Assay | 320 nM | 5 d | DMSO | Inhibition of GCS-F induced granulocytic differentiation | 25805962 |
| SET-2 | Cytotoxic Assay | 5 μM | 48 h | Cytotoxic index=18.7% | 25931349 | |
| HEL | Cytotoxic Assay | 5 μM | 48 h | Cytotoxic index=12.2% | 25931349 | |
| Human monocyte | Kinase Assay | Inhibition of JAK2/1 in human monocytes expressing CD14 assessed as inhibition of IFNgamma-stimulated STAT1 phosphorylation with IC50 of 0.031μM | 23540648 | |||
| Human monocyte | Kinase Assay | Inhibition of JAK2 in human monocytes expressing CD14 assessed as inhibition of GM-CSF-stimulated STAT5a phosphorylation with IC50 of 0.026μM | 23540648 | |||
| Human T cell | Kinase Assay | Inhibition of JAK3/1 in human T cells expressing CD3 assessed as inhibition of IL2-stimulated STAT5a phosphorylation with IC50 of 0.023μM | 23540648 | |||
| TF1 | Kinase Assay | 20 min | DMSO | Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation with IC50 of 0.024μM | 22698084 | |
| TF1 | Kinase Assay | 20 min | DMSO | Inhibition of JAK2 in human TF1 cells assessed as inhibition of EPO-induced STAT5 phosphorylation with IC50 of 0.012μM | 22698084 | |
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0001 μM | 23668484 | ||
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0002 μM | 23668484 | ||
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0005 μM | 23668484 | ||
| SET2 cells | JAK inhibition assay | IC50 = 0.00184 μM | 23061660 | |||
| Sf21 cells | JAK inhibition assay | 1 h | IC50 = 0.0028 μM | 22591402 | ||
| Sf21 cells | JAK inhibition assay | 60 min | IC50 = 0.003 μM | 27137359 | ||
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0032 μM | 23668484 | ||
| Sf21 cells | JAK inhibition assay | 1 h | IC50 = 0.0033 μM | 22591402 | ||
| TF1 cells | JAK inhibition assay | 30 min | IC50 = 0.00685 μM | 23061660 | ||
| CD34+ cells | JAK inhibition assay | IC50 = 0.008 μM | 26927423 | |||
| TF1 cells | JAK inhibition assay | 20 min | EC50 = 0.012 μM | 22698084 | ||
| Sf21 cells | TYK2 inhibition assay | 1 h | IC50 = 0.019 μM | 22591402 | ||
| T cells | JAK inhibition assay | IC50 = 0.023 μM | 23540648 | |||
| T cells | JAK inhibition assay | IC50 = 0.023 μM | 23540648 | |||
| TF1 cells | JAK inhibition assay | 20 min | EC50 = 0.024 μM | 22698084 | ||
| T cells | JAK inhibition assay | IC50 = 0.031 μM | 23540648 | |||
| T cells | JAK inhibition assay | IC50 = 0.031 μM | 23540648 | |||
| PBMC cells | JAK inhibition assay | IC50 = 0.04 μM | 26927423 | |||
| Sf21 cells | JAK inhibition assay | 1 h | IC50 = 0.428 μM | 22591402 | ||
| PBMC cells | STAT5 inhibition assay | IC50 = 0.448 μM | 26927423 | |||
| CD34+ cells | JAK inhibition assay | 45 min | IC50 = 0.677 μM | 24417533 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 300 mg/mL
(979.2 mM)
Ethanol : 12 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 306.37 | Formula | C17H18N6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 941678-49-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | INCB018424 | Smiles | C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3 | ||
Mechanism of Action
| Targets/IC50/Ki |
JAK2
(Cell-free assay) 2.8 nM
JAK1
(Cell-free assay) 3.3 nM
|
|---|---|
| In vitro |
Ruxolitinib (INCB18424) potently and selectively inhibits JAK2V617F-mediated signaling and proliferation in Ba/F3 cells and HEL cells. This compound markedly increases apoptosis in a dose dependent manner in Ba/F3 cells. It (64 nM) results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. This chemical inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 of 407 nM and 223 nM, respectively. It demonstrates remarkable potency against erythroid colony formation with IC50 of 67nM.
|
| Kinase Assay |
Binding assay
|
|
Recombinant proteins are expressed using Sf21 cells and baculovirus vectors and purified with affinity chromatography. JAK kinase assays use a homogeneous time-resolved fluorescence assay with the peptide substrate (-EQEDEPEGDYFEWLE). Each enzyme reaction is carried out with Ruxolitinib (INCB18424) or control, JAK enzyme, 500 nM peptide, adenosine triphosphate (ATP; 1mM), and 2% dimethyl sulfoxide (DMSO) for 1 hour. The 50% inhibitory concentration (IC50) is calculated as the concentration of this compound required for inhibition of 50% of the fluorescent signal.
|
|
| In vivo |
INCB018424 (180 mg/kg, orally, twice a day) results in a survival rate of greater than 90% by day 22 in a JAK2V617F-driven mouse model. This compound (180 mg/kg, orally, twice a day) markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminates neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model. In the double-blind trial of myelofibrosis, the primary end point is reached in 41.9% of patients in the Ruxolitinib (INCB18424) group as compared with 0.7% in the placebo group. It results in maintaining reduction in spleen volume and improvement of 50% or more in the total symptom score. A total of 28% of the patients in the group receiving this compound (15 mg twice daily) has at least a 35% reduction in spleen volume at week 48 in patients with myelofibrosis, as compared with 0% in the group receiving the best available therapy. The mean palpable spleen length has decreased by 56% with it but has increased by 4% with the best available therapy at week 48. Patients in the ruxolitinib group has an improvement in overall quality-of-life measures and a reduction in symptoms associated with myelofibrosis.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
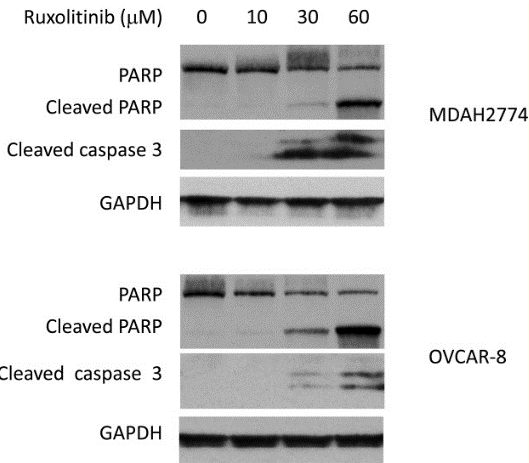
| Western blot | cleaved PARP / cleaved caspase3 p-JAK2 / p-AKT / p-MAPK / Bcl-xl / MCL-1 c-Myc / c-Jun / Cyclin B / Cyclin D / Bcl-2 / HIF-1α p-STAT3 |
|
29849942 |
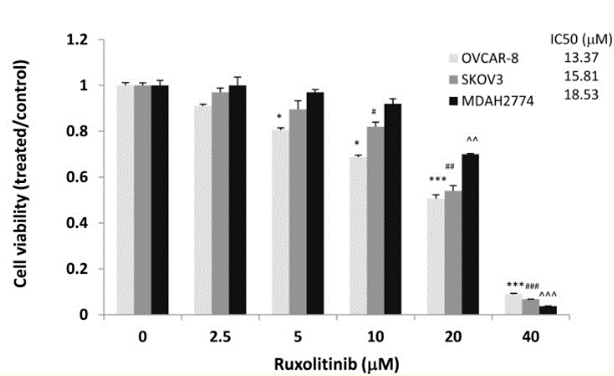
| Growth inhibition assay | Cell viability Cell apoptosis Cell proliferation |
|
29849942 |
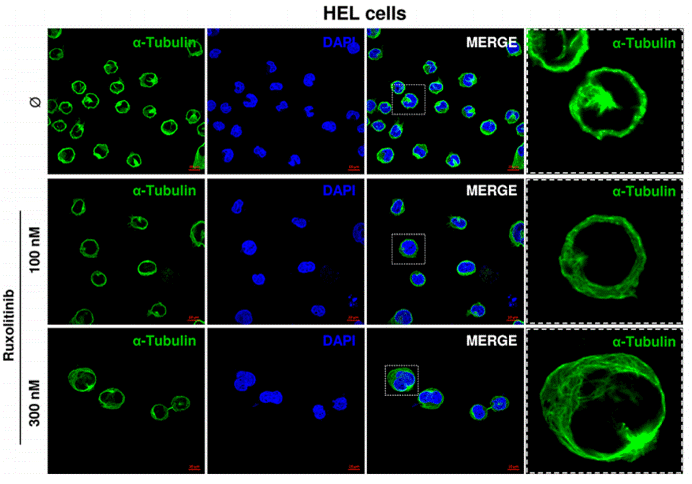
| Immunofluorescence | α-tubulin |
|
26356819 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06397313 | Not yet recruiting | Myelofibrosis |
Ryvu Therapeutics SA |
September 2024 | Phase 2 |
| NCT06388564 | Not yet recruiting | Chronic Graft-versus-host-disease |
Incyte Corporation |
July 8 2024 | Phase 2 |
| NCT06251102 | Not yet recruiting | Polycythemia Vera |
Gruppo Italiano Malattie EMatologiche dell''Adulto |
July 2024 | -- |
| NCT06343792 | Not yet recruiting | Steroid Refractory GVHD |
ReAlta Life Sciences Inc. |
May 2024 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
What is the difference between S2902 and S1378 which seem to have same structure formula according to the product information?
Answer:
These two chemicals are the two different chiral forms of this compound. S2902 S-Ruxolitinib is the S form and S1378 Ruxolitinib is the D form. One of the carbon atoms in it is asymmetric, making the two molecules mirror images of each other. The biological activities of these two molecules can be very different because of the confirmation differences.
Question 2:
How about the half-life of this compound? How long is the duration of its inhibitory effect on JAK-STAT signaling?
Answer:
According to previous study, the half-life of this compound in body is about 2~3 hours. Generally, it is longer in vitro culture medium than in vivo. It was also used for 24 hours in paper. http://www.bloodjournal.org/cgi/pmidlookup?view=long&pmid=24711661.






































