- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
Rucaparib phosphate PARP inhibitor
Rucaparib phosphate is an inhibitor of PARP with Ki of 1.4 nM for PARP1 in a cell-free assay, also showing binding affinity to eight other PARP domains. Phase 3.
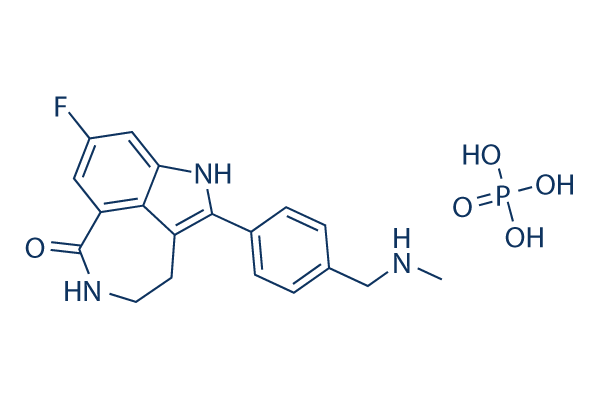
Chemical Structure
Molecular Weight: 421.36
Purity & Quality Control
Batch:
Purity:
99.89%
99.89
Related Products
| Related Targets | PARP1 PARP2 PARP3 Tankyrase-1 Tankyrase-2 PARP14 PARP7 TNKS1 TNKS2 | Click to Expand |
|---|---|---|
| Related Products | XAV-939 Veliparib (ABT-888) PJ34 HCl AG-14361 Iniparib (BSI-201) A-966492 G007-LK UPF 1069 AZD2461 Pamiparib ME0328 3-Aminobenzamide NMS-P118 Stenoparib (E7449) NVP-TNKS656 WIKI4 Benzamide BGP-15 2HCl NU1025 BYK204165 | Click to Expand |
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Apoptosis Compound Library DNA Damage/DNA Repair compound Library Cell Cycle compound library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| BT-474 | Function Assay | 0.1/1/500/1000 nM | inhibits PARP activity at starting concerntration of 500 nM | 25128455 | ||
| BT474 | Growth Inhibition Assay | 500 nM | 10–15 d | reduces cell growth in the four lines and significantly | 25128455 | |
| SKBR3 | Growth Inhibition Assay | 500 nM | 10–15 d | reduces cell growth in the four lines and significantly | 25128455 | |
| AU565 | Growth Inhibition Assay | 500 nM | 10–15 d | reduces cell growth in the four lines and significantly | 25128455 | |
| EFM192A | Growth Inhibition Assay | 500 nM | 10–15 d | reduces cell growth in the four lines and significantly | 25128455 | |
| MDA-MB-231 | Function Assay | 10/20/40 μM | 24 h | increases p-AKT levels in a dose-dependent manner | 24420152 | |
| MDA-MB-231 | Cell Viability Assay | 0.1-40 μM | 24 h | IC50 = 17.77 μM | 24420152 | |
| MDA-MB-231 | Apoptosis Assay | 10/20/40 μM | 24 h | induces apoptosis dose dependently | 24420152 | |
| MDA-MB-231 | Growth Inhibition Assay | 10/20/40 μM | 24 h | blocks cell cycle progression in G2/M phase | 24420152 | |
| H460 | Growth Inhibition Assay | 400 nM | 24 h | increases cellular radiosensitivity | 24411611 | |
| A549 | Growth Inhibition Assay | 400 nM | 24 h | increases cellular radiosensitivity | 24411611 | |
| DT40 | Growth Inhibition Assay | IC50=21 nM | 24356813 | |||
| DU145 | Growth Inhibition Assay | IC50=18 nM | 24356813 | |||
| COLO704 | Growth Inhibition Assay | IC50=2.52 ± 0.67 μM | 23729402 | |||
| OVMANAb | Growth Inhibition Assay | IC50=2.58 ± 0.38 μM | 23729402 | |||
| OV177 | Growth Inhibition Assay | IC50=2.78 ± 0.71 μM | 23729402 | |||
| OAW28 | Growth Inhibition Assay | IC50=3.61 ± 0.28 μM | 23729402 | |||
| OVSAHO | Growth Inhibition Assay | IC50=3.64 ± 0.33 μM | 23729402 | |||
| OVKATE | Growth Inhibition Assay | IC50=3.64 ± 1.79 μM | 23729402 | |||
| OVCAR3 | Growth Inhibition Assay | IC50=3.74 ± 0.40 μM | 23729402 | |||
| PEO14 | Growth Inhibition Assay | IC50=3.84 ± 0.76 μM | 23729402 | |||
| A2780 | Growth Inhibition Assay | IC50=3.94 ± 0.25 μM | 23729402 | |||
| OVTOKO | Growth Inhibition Assay | IC50=4.14 ± 1.53 μM | 23729402 | |||
| KURAMOCHIb | Growth Inhibition Assay | IC50=4.34 ± 0.29 μM | 23729402 | |||
| TOV21G | Growth Inhibition Assay | IC50=5.07 ± 1.30 μM | 23729402 | |||
| OVISE | Growth Inhibition Assay | IC50=5.68 ± 0.23 μM | 23729402 | |||
| KK | Growth Inhibition Assay | IC50=6.15 ± 1.42 μM | 23729402 | |||
| RMUGS | Growth Inhibition Assay | IC50=7.03 ± 1.83 μM | 23729402 | |||
| PEO6 | Growth Inhibition Assay | IC50=7.06 ± 0.74 μM | 23729402 | |||
| OVCA429 | Growth Inhibition Assay | IC50=8.29 ± 1.64 μM | 23729402 | |||
| OV167 | Growth Inhibition Assay | IC50=8.33 ± 1.18 μM | 23729402 | |||
| RMG1 | Growth Inhibition Assay | IC50=9.32 ± 2.36 μM | 23729402 | |||
| OVCAR5 | Growth Inhibition Assay | IC50=9.50 ± 2.59 μM | 23729402 | |||
| EFO21 | Growth Inhibition Assay | IC50=9.92 ± 1.87 μM | 23729402 | |||
| ES2 | Growth Inhibition Assay | IC50=10.12 ± 1.23 μM | 23729402 | |||
| Tyk-nu | Growth Inhibition Assay | IC50=10.20 ± 1.12 μM | 23729402 | |||
| CAOV3 | Growth Inhibition Assay | IC50=10.37 ± 0.87 μM | 23729402 | |||
| OV207 | Growth Inhibition Assay | IC50=12.27 ± 0.32 μM | 23729402 | |||
| HEY | Growth Inhibition Assay | IC50=13.01 ± 0.75 μM | 23729402 | |||
| DOV13 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| EFO27 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| HEY C2 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| KOC-7cc | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| MCASb | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| OAW42 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| OV2008 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| OV90 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| OVCA420b | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| OVCA432 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| PEA2 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| SKOV3 | Growth Inhibition Assay | IC50>15 μM | 23729402 | |||
| TOV112D | Growth Inhibition Assay | 0-3 μM | IC50>15 μM | 23729402 | ||
| C4-2 | Growth Inhibition Assay | 0-3 μM | 14 d | DMSO | decreases colony number dose dependently | 23565244 |
| PC3 | Growth Inhibition Assay | 0-3 μM | 14 d | DMSO | decreases colony number dose dependently | 23565244 |
| DU145 | Growth Inhibition Assay | 0-3 μM | 14 d | DMSO | decreases colony number dose dependently | 23565244 |
| VCaP | Growth Inhibition Assay | 0-3 μM | 14 d | DMSO | decreases colony number dose dependently | 23565244 |
| LNCaP | Growth Inhibition Assay | 0-3 μM | 14 d | DMSO | decreases colony number dose dependently | 23565244 |
| MDA-MB-468 | Cell Viability Assay | IC50=9.7 μM | 22678161 | |||
| MDA-MB-231 | Cell Viability Assay | IC50=13 μM | 22678161 | |||
| Cal-51 | Cell Viability Assay | IC50=8.6 μM | 22678161 | |||
| LoVo | Function assay | 30 mins | Inhibition of PARP in human LoVo cells assessed as inhibition of poly(ADP)-ribose polymerization for 30 mins by fluorescence assay, EC50 = 0.00469 μM. | 26652717 | ||
| MX1 | Cytotoxicity assay | Cytotoxicity against BRCA1-deficient human MX1 cells, EC50 = 0.0053 μM. | 26652717 | |||
| Rosetta2 (DE3) | Function assay | Inhibition of human N-terminal 6xhis-tagged ARTD6 (873 to 1161) expressed in Escherichia coli Rosetta2 (DE3) cells using NAD+ as substrate by fluorescence assay, IC50 = 0.014 μM. | 24900770 | |||
| Rosetta2 (DE3) | Function assay | Inhibition of human 6xhis-tagged ARTD5 (1030 to 1317) expressed in Escherichia coli Rosetta2 (DE3) cells using NAD+ as substrate by fluorescence assay, IC50 = 0.025 μM. | 24900770 | |||
| LoVo | Cytotoxicity assay | 0.4 uM | 5 days | Potentiation of temozolomide-induced cytotoxicity in human LoVo cells assessed as temozolomide GI50 at 0.4 uM after 5 days by Celltiter-Glo assay, GI50 = 0.144 μM. | 26652717 | |
| Capan1 | Cytotoxicity assay | Cytotoxicity against BRCA2-deficient human Capan1 cells, EC50 = 0.609 μM. | 26652717 | |||
| MDA-MB-436 | Anticancer assay | 96 hrs | Anticancer activity against human BRCA1-deficient MDA-MB-436 cells after 96 hrs by MTT assay, CC50 = 3 μM. | 26342868 | ||
| OVCAR3 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human OVCAR3 cells after 24 hrs by MTT assay, IC50 = 3.31 μM. | 29456106 | ||
| MRC5 | Cytotoxicity assay | Cytotoxicity against human MRC5 cells, EC50 = 8.53 μM. | 26652717 | |||
| MCF7 | Anticancer assay | 96 hrs | Anticancer activity against human MCF7 cells after 96 hrs by MTT assay, CC50 = 19.47 μM. | 26342868 | ||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Features | The first PARP inhibitor used in clinical trials combined with temozolomide. | ||
|---|---|---|---|
| Targets |
|
In vitro |
||||
| In vitro | Rucaparib is a potent inhibitor of purified full-length human PARP-1 and shows higher inhibition of cellular PARP in LoVo and SW620 cells. Besides, Rucaparib binds detectably to eight other PARP domains, including PARP2, 3, 4, 10, 15, 16, TNKS1 and TNKS2. [1] [2] The radio-sensitization by Rucaparib is due to downstream inhibition of activation of NF-κB, and is independent of SSB repair inhibition. Rucaparib could target NF-κB activated by DNA damage and overcome toxicity observed with classical NF-κB inhibitors without compromising other vital inflammatory functions. [3] Rucaparib inhibits PARP-1 activity by 97.1% at a concentration of 1 μM in permeabilised D283Med cells. [4] | |||
|---|---|---|---|---|
| Kinase Assay | Ki Determination | |||
| Inhibition of human full-length recombinant PARP-1 by [32P]NAD+ incorporation is measured. The [32P]ADP-ribose incorporated into acid insoluble material is quantified using a PhosphorImager. Ki is calculated by nonlinear regression analysis. | ||||
| Cell Research | Cell lines | D425Med, D283Med and D384Med cells | ||
| Concentrations | 0.4 μM | |||
| Incubation Time | 3 or 5 days | |||
| Method | Medulloblastoma cell lines are seeded in 96-well plates at a density of 1 × 103, 3 × 103 and 3 × 103, respectively. At 24 hours (D384Med) or 48 hours (D283Med and D425Med) after seeding, the cells are exposed to various concentrations of temozolomide in the presence or absence of 0.4 μM Rucaparib. After 3 days (D425Med and D384Med) or 5 days (D283Med) of culture, cell viability is evaluated by a XTT cell proliferation kit assay. Cell growth is expressed as a percentage in relation to DMSO or 0.4 μM Rucaparib-alone controls. The concentration of temozolomide, alone or in combination with Rucaparib that inhibited growth by 50% (GI50) is calculated. The potentiation factor 50 (PF50) is defined as the ratio of the GI50 of temozolomide in the presence of Rucaparib to the GI50 of temozolomide alone. | |||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | PAR BRCA1 |
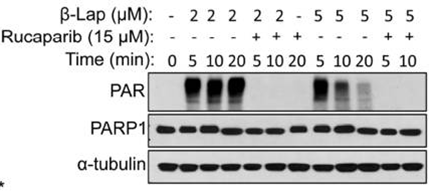
|
27960087 | |
| Growth inhibition assay | Cell viability |
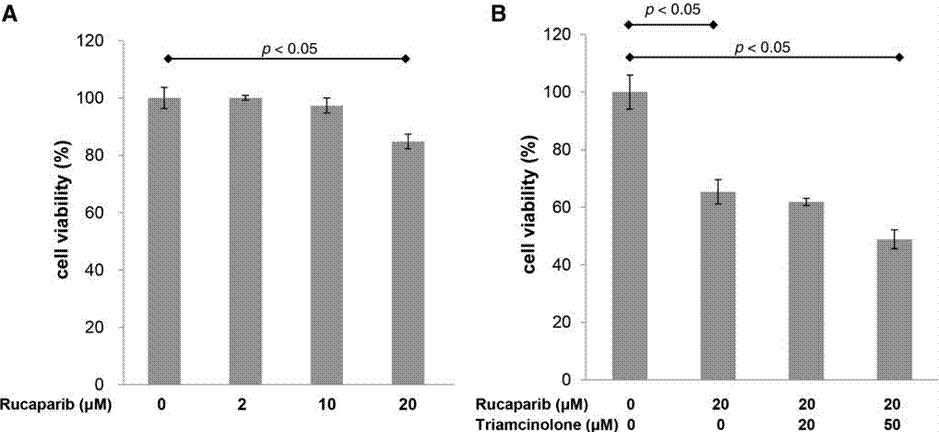
|
31119062 | |
| Immunofluorescence | α-tubulin PAR γH2AX 53BP1 |
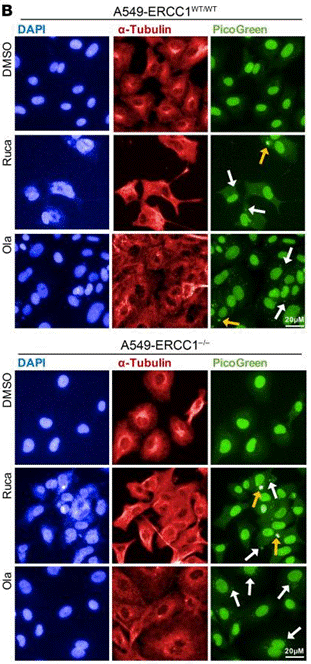
|
30589644 | |
In Vivo |
||
| In vivo | Rucaparib is not toxic but significantly enhances temozolomide-induced TGD in the DNA repair protein-competent D384Med xenografts. Pharmacokinetics studies also show that Rucaparib is detected in the brain tissue, which indicates that Rucaparib has potential in intra-cranial malignancy therapy. [4] Rucaparib significantly potentiates the cytotoxicity of topotecan and temozolomide in NB-1691, SH-SY-5Y, and SKNBE (2c) cells. Rucaparib enhances the antitumor activity of temozolomide and indicates complete and sustained tumor regression in NB1691 and SHSY5Y xenografts. [5] | |
|---|---|---|
| Animal Research | Animal Models | CD-1 nude mice bearing established D283Med xenografts |
| Dosages | 1 mg/kg | |
| Administration | One or four daily by i.p. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04539327 | Completed | Epithelial Ovarian Cancer|Fallopian Tube Cancer|Primary Peritoneal Cancer |
Grupo Español de Investigación en Cáncer de Ovario|Clovis Oncology Inc. |
July 29 2020 | -- |
| NCT04209595 | Active not recruiting | Small Cell Lung Cancer|Extra-Pulmonary Small Cell Carcinomas |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
April 8 2020 | Phase 1|Phase 2 |
| NCT04179396 | Completed | Metastatic Castration Resistant Prostate Cancer |
pharmaand GmbH |
December 5 2019 | Phase 1 |
| NCT03824704 | Terminated | Epithelial Ovarian Cancer|Fallopian Tube Cancer|Primary Peritoneal Carcinoma|High Grade Serous Carcinoma|Endometrioid Adenocarcinoma |
pharmaand GmbH|Bristol-Myers Squibb|Foundation Medicine |
August 23 2019 | Phase 2 |
| NCT03840200 | Completed | Breast Cancer|Prostate Cancer|Ovarian Cancer |
Hoffmann-La Roche |
June 12 2019 | Phase 1 |
References |
|
Chemical Information
| Molecular Weight | 421.36 | Formula | C19H18FN3O.H3PO4 |
| CAS No. | 459868-92-9 | SDF | Download SDF |
| Synonyms | AG-014699 phosphate, PF-01367338 phosphate | ||
| Smiles | CNCC1=CC=C(C=C1)C2=C3CCNC(=O)C4=C3C(=CC(=C4)F)N2.OP(=O)(O)O | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 85 mg/mL ( (201.72 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : 7 mg/mL Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field