research use only
OC000459 GPR antagonist
Cat.No.S2822
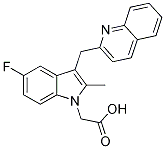
Chemical Structure
Molecular Weight: 348.37
Quality Control
Solubility
|
In vitro |
DMSO
: 4 mg/mL
(11.48 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 348.37 | Formula | C21H17FN2O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 851723-84-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1=C(C2=C(N1CC(=O)O)C=CC(=C2)F)CC3=NC4=CC=CC=C4C=C3 | ||
Mechanism of Action
| Targets/IC50/Ki |
DP2
13 nM
|
|---|---|
| In vitro |
OC000459 inhibits the binding of [3H]PGD2 to membranes from CHO cells transfected with human DP2 with Ki of 13 nM. This compound also displaces [3H]PGD2 from membranes from human Th2 lymphocytes with Ki of 4 nM. It antagonizes PGD2-mediated calcium mobilization in a concentration-dependent manner with IC50 of 28 nM in intact CHO cells expressing DP2. This chemical inhibits chemotaxis of human Th2 cells in response to PGD2 (10 nM) with IC50 of 28 nM. It antagonizes the effect of PGD2 competitively in both the isolated leukocyte preparation and heparinized human whole blood. This compound inhibits eosinophil shape change responses to DK-PGD2 with IC50 of 11 nM. It inhibits the activation of Th2 cells and eosinophils in response to mast cell supernatants.
|
| In vivo |
OC000459 administrated at doses of 2 mg/kg p.o. in the Sprague-Dawley rats shows plasma half-life of 2.9 hours, time that maximal plasma concentration is achieved of 1.3 hours, maximal plasma concentration achieved is 1.54 μg/mL. This compound orally administrated 0.5 hour before injection of DK-PGD2 leads to a dose-dependent reduction in blood eosinophilia with ED50 of 0.04 mg/kg in rats. It orally administrated 0.5 hour before injection of DK-PGD2 also leads to a dose-dependent inhibition eosinophil accumulation ED50 of 0.01 mg/kg in rats. This chemical (200 mg twice daily for 28 days) administrated in patients with moderate persistent asthma shows improvement in quality of life as analysed for both the Full Analysis (FA) population and the Per Protocol (PP) population. In those patients, it improves the night-time symptom scores, reduces the geometric mean sputum eosinophil count and respiratory infections. This compound (200 mg twice daily) treatment inhibits the later asthmatic responses and the post allergen increase in sputum eosinophils in steroid naive asthmatic patients.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02560610 | Completed | Severe Eosinophilic Asthma |
Chiesi Farmaceutici S.p.A.|Atopix Therapeutics Ltd. |
September 2016 | Phase 2 |
| NCT01056783 | Completed | Eosinophilic Esophagitis |
Oxagen Ltd |
August 2010 | Phase 2 |
| NCT01056575 | Completed | Healthy Volunteers |
Oxagen Ltd |
February 2010 | Phase 1 |
| NCT01448902 | Completed | Allergic Rhinitis |
Oxagen Ltd |
March 2007 | Phase 2 |
| NCT00290381 | Completed | Allergic Rhinitis |
Trevor Hansel|Oxagen Ltd|Imperial College London |
Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































