
- Inhibitors
- By product type
- Natural Products
- Inducing Agents
- Peptides
- Antibiotics
- Antibody-drug Conjugates(ADC)
- PROTAC
- Hydrotropic Agents
- Dyes
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
By research - Antibodies
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- Featured Products
- MRTX1133
- Nab-Paclitaxel
- KP-457
- IAG933
- RMC-6236 (Daraxonrasib)
- RMC-7977
- Zoldonrasib (RMC-9805)
- GsMTx4
- Navitoclax (ABT-263)
- TSA (Trichostatin A)
- Y-27632 Dihydrochloride
- SB431542
- SB202190
- MK-2206 Dihydrochloride
- LY294002
- Alisertib (MLN8237)
- XAV-939
- CHIR-99021 (Laduviglusib)
- Bafilomycin A1 (Baf-A1)
- Thiazovivin (TZV)
- CP-673451
- Verteporfin
- DAPT
- Galunisertib (LY2157299)
- MG132
- SBE-β-CD
- Tween 80
- Bavdegalutamide (ARV-110)
- Z-VAD-FMK
- Wnt-C59 (C59)
- IWR-1-endo
- (+)-JQ1
- 3-Deazaneplanocin A (DZNep) Hydrochloride
- RepSox (E-616452)
- Erastin
- Q-VD-Oph
- Puromycin Dihydrochloride
- Cycloheximide
- Telaglenastat (CB-839)
- A-83-01
- Ceralasertib (AZD6738)
- Liproxstatin-1
- Emricasan (IDN-6556)
- PMA (Phorbol 12-myristate 13-acetate)
- Dibutyryl cAMP (Bucladesine) sodium
- Nedisertib (M3814)
- PLX5622
- IKE (Imidazole Ketone Erastin)
- STM2457
- Saruparib (AZD5305)
- New Products
- Contact Us
research use only
I-BET151 (GSK1210151A) Epigenetic Reader Domain inhibitor
Cat.No.S2780
Chemical Structure
Molecular Weight: 415.44
Quality Control
Batch:
Purity:
99.99%
99.99
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MV4;11 | cytotoxicity assay | ~100 μM | DMSO | IC50=26 nM | 21964340 | |
| RS4;11 | cytotoxicity assay | ~100 μM | DMSO | IC50=192 nM | 21964340 | |
| MOLM13 | cytotoxicity assay | ~100 μM | DMSO | IC50=120 nM | 21964340 | |
| NOMO1 | cytotoxicity assay | ~100 μM | DMSO | IC50=15 nM | 21964340 | |
| HEL | cytotoxicity assay | ~100 μM | DMSO | IC50=1 μM | 21964340 | |
| K562 | cytotoxicity assay | ~100 μM | DMSO | IC50>100 μM | 21964340 | |
| MEG01 | cytotoxicity assay | ~100 μM | DMSO | IC50=25 μM | 21964340 | |
| HL60 | cytotoxicity assay | ~100 μM | DMSO | IC50=890 nM | 21964340 | |
| MV4;11 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 21964340 | |
| MOLM13 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 21964340 | |
| MV4;11 | Function assay | DMSO | decreases the recruitment of BRD3/4 and impaired recruitment of CDK9 and PAF1 to the transcriptional start site | 21964340 | ||
| PBMC | Function assay | DMSO | inhibits IL-6 with pIC50 of 6.7 | 22437115 | ||
| A2 | Function assay | ~10 μM | DMSO | reactivates latent HIV-1 | 23255218 | |
| A72 | Function assay | ~10 μM | DMSO | reactivates latent HIV-1 | 23255218 | |
| BC1 | Growth inhibitory assay | ~1 μM | DMSO | IC50=220 nM | 23792448 | |
| BC3 | Growth inhibitory assay | ~1 μM | DMSO | IC50=460 nM | 23792448 | |
| BCBL1 | Growth inhibitory assay | ~1 μM | DMSO | IC50=330 nM | 23792448 | |
| BJAB | Growth inhibitory assay | ~1 μM | DMSO | IC50=970 nM | 23792448 | |
| Namalwa | Growth inhibitory assay | ~1 μM | DMSO | IC50=970 nM | 23792448 | |
| Jurkat | Growth inhibitory assay | ~1 μM | DMSO | IC50=1220 nM | 23792448 | |
| MM1S | Growth inhibitory assay | ~1 μM | DMSO | IC50=760 nM | 23792448 | |
| U266 | Growth inhibitory assay | ~1 μM | DMSO | IC50=950 nM | 23792448 | |
| UM-PEL-1 | Growth inhibitory assay | ~1 μM | DMSO | IC50=210 nM | 23792448 | |
| UM-PEL-3 | Growth inhibitory assay | ~1 μM | DMSO | IC50=180 nM | 23792448 | |
| BC1 | Function assay | 500 nM | DMSO | induces cell-cycle arrest | 23792448 | |
| BC3 | Function assay | 500 nM | DMSO | induces cell-cycle arrest | 23792448 | |
| BC1 | Function assay | 800 nM | DMSO | reduces c-Myc protein levels | 23792448 | |
| BC3 | Function assay | 800 nM | DMSO | reduces c-Myc protein levels | 23792448 | |
| H929 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS12PE | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS12BM | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS18 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS11 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| RPMI8226 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| H929 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS12PE | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS12BM | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS18 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS11 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| RPMI8226 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| U87MG | Function assay | ~10 μM | DMSO | reduces U87MG cellular ATP with IC50 of 1.05 μM | 24496381 | |
| A172 | Function assay | ~10 μM | DMSO | reduces cellular ATP with IC50 of 1.28 μM | 24496381 | |
| SW1783 | Function assay | ~10 μM | DMSO | reduces cellular ATP with IC50 of 2.68 μM | 24496381 | |
| U87MG | Function assay | ~10 μM | DMSO | increases proportion of cells in the G1/S transition | 24496381 | |
| RAW267.4 | Function assay | 1 μM | DMSO | reduces IL-6 production induced by LPS | 24859008 | |
| RAW267.4 | Function assay | 1 μM | DMSO | reduces the association between BRD4 and acetylated p65 | 24859008 | |
| Me007 | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| SK-Mel-28 | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Mel-RMU | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Mel-JD | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Mel-RM | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Me007 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| SK-Mel-28 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Mel-RMU | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Mel-JD | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Mel-RM | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Me007 | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| SK-Mel-28 | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Mel-RMU | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Mel-JD | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Mel-RM | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Me007 | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| SK-Mel-28 | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| Mel-RMU | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| Mel-JD | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| Mel-RM | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| HepG2 | Function assay | 18 hrs | Upregulation of ApoA1 expression in human HepG2 cells assessed as concentration required to increase 70% of luciferase activity after 18 hrs by luciferase reporter gene assay, EC170 = 0.09 μM. | 22386529 | ||
| Raji | Function assay | 4 hrs | Inhibition of BRD4 in human Raji cells assessed as reduction of MYC expression after 4 hrs, IC50 = 0.13 μM. | 24900758 | ||
| MV4-11 | Growth inhibition assay | 72 hrs | Growth inhibition of human MV4-11 cells after 72 hrs by SRB assay, IC50 = 0.119 μM. | 25559428 | ||
| MM1S | Growth inhibition assay | 72 hrs | Growth inhibition of human MM1S cells after 72 hrs by SRB assay, IC50 = 0.299 μM. | 25559428 | ||
| HT-29 | Growth inhibition assay | 72 hrs | Growth inhibition of human HT-29 cells after 72 hrs by SRB assay, IC50 = 0.945 μM. | 25559428 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD1 (44 to 168 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, IC50 = 0.0317 μM. | 26080064 | ||
| MV4-11 | Cytotoxicity assay | 4 days | Cytotoxicity against human MV4-11 cells harboring MLL1 fusion gene assessed as growth inhibition after 4 days by CellTiter-Glo luminescent assay, IC50 = 0.162 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD2 (333 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, IC50 = 0.226 μM. | 26080064 | ||
| MOLM13 | Cytotoxicity assay | 4 days | Cytotoxicity against human MOLM13 cells harboring MLL1 fusion gene assessed as growth inhibition after 4 days by CellTiter-Glo luminescent assay, IC50 = 0.228 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, IC50 = 0.0317 μM. | 28463487 | ||
| MV4-11 | Growth inhibition assay | 4 days | Growth inhibition of human MV4-11 cells after 4 days by WST-8 assay, IC50 = 0.162 μM. | 28463487 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, IC50 = 0.226 μM. | 28463487 | ||
| MOLM13 | Growth inhibition assay | 4 days | Growth inhibition of human MOLM13 cells after 4 days by WST-8 assay, IC50 = 0.228 μM. | 28463487 | ||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD3 BD1 (24 to 144 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0298 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD3 BD2 (306 to 417 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0405 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD4 BD1 (44 to 168 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0528 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD2 BD1 (72 to 205 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0548 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD2 BD2 (349 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0703 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD4 BD2 (333 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.215 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated CREBBP (1043 to 1159 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 3.084 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD3 BD1 (24 to 144 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0072 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD1 (44 to 168 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.009 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD2 BD1 (72 to 205 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.009 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD3 BD2 (306 to 417 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0223 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD2 BD2 (349 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0496 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD2 (333 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0748 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, Ki = 0.009 μM. | 28463487 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, Ki = 0.0748 μM. | 28463487 | ||
| THP1 | Antiinflammatory assay | Antiinflammatory activity in human THP1 cells | 22386529 | |||
| HT-29 | Function assay | 0.3125 uM to 5 uM | 24 hrs | Inhibition of BRD4 in human HT-29 cells assessed as reduction in c-Myc protein expression at 0.3125 uM to 5 uM uM after 24 hrs by Western blotting method | 25559428 | |
| MV4-11 | Function assay | Inhibition of BRD4 in human MV4-11 cells assessed as downregulation of BCL2 RNA expression by RNA-seq analysis | 29259751 | |||
| MV4-11 | Function assay | Inhibition of BRD4 in human MV4-11 cells assessed as downregulation of cMYC RNA expression by RNA-seq analysis | 29259751 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| fibroblast cells | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for control Hh wild type fibroblast cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Chemical Information, Storage & Stability
| Molecular Weight | 415.44 | Formula | C23H21N5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1300031-49-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1=C(C(=NO1)C)C2=C(C=C3C(=C2)N=CC4=C3N(C(=O)N4)C(C)C5=CC=CC=N5)OC | ||
Solubility
|
In vitro |
DMSO
: 83 mg/mL
(199.78 mM)
Ethanol : 83 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Features |
Optimized to retain excellent BET target potency and selectivity while enhancing the in vivo pharmacokinetics and terminal half-life to enable prolonged in vivo studies.
|
|---|---|
| Targets/IC50/Ki | |
| In vitro |
I-BET151 (GSK1210151A) exhibits potent selectivity over an extensive range of diverse protein types such as COX-2, P450, Aurora B, GSK3β, PI3K-γ, GPCR, ion channels, and transporters. Similar to I-BET762 (GSK525762A), it displays potent binding affinity to BRD2, BRD3 and BRD4 with KD of 0.02-0.1 μM, and significantly inhibits lipopolysaccharide-stimulated IL-6 cytokine production in human peripheral blood mononuclear cells (PBMC) and whole blood (WB) as well as rat WB with IC50 of 0.16 μM, 1.26 μM, and 1.26 μM, respectively. This compound (0.5 or 5 μM) inhibits the binding of BETs (BRD2, BRD3, BRD4, and BRD9) but not the binding of 23 other bromodomain proteins in HL60 nuclear extract to acetylated histone peptides. It has potent efficacy against cell lines harboring different MLL-fusions such as MV4;11, RS4;11, MOLM13, and NOMO1 cells with IC50 of 15-192 nM. Consistently, it completely ablates the colony-forming potential of MLL-fusion-driven leukaemias (MOLM13) but not leukaemias driven by tyrosine kinase activation (K562). I-BET151 also displays potent efficacy in both liquid culture and clonogenic assays using primary murine progenitors transformed with either MLL-ENL or MLL-AF9. Treatment with this compound significantly induces apoptosis and prominent G0/G1 arrest in MLL-fusion cell lines driven by distinct MLL fusions (MOLM13 and MV4;11 containing MLL-AF9 and MLL-AF4, respectively) but not the K562 cells, probably due to the inhibition of transcription of BCL2, C-MYC and CDK6 by blocking the recruitment of BRD3/4, PAFc and SEC components into transcriptional start site (TSS). [1]
|
| Kinase Assay |
Fluorescence anisotropy (FP) ligand displacement assay
|
|
All components are dissolved in buffer of composition 50 mM HEPES pH 7.4, 150 mM NaCl and 0.5 mM CHAPS with final concentrations of BRD 2/3/4 75 nM, fluorescent ligand 5 nM. 10 μL of this reaction mixture is added using a micro multidrop to wells containing 100 nL of various concentrations of I-BET151 (GSK1210151A) or DMSO vehicle (1% final) in Greiner 384 well Black low volume microtitre plate and equilibrated in the dark for 60 minutes at room temperature. Fluorescence anisotropy is read in Envision (lex = 485 nm, lEM = 530 nm; Dichroic = 505 nM).
|
|
| In vivo |
I-BET151 (GSK1210151A), when administered at 30 mg/kg/day, significantly inhibits tumor growth of murine MLL-AF9 and human MLL-AF4 leukaemia in mice, and provides marked survival benefit. [1]
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | α-SMA / Fibronectin / Collagen-1 FoxM1 / AURKB / Survivin / cyclin B / PLK1 HP1α / HP1β / HP1γ |
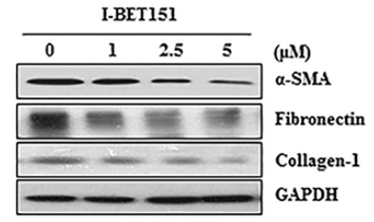
|
27732564 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































