- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
GSK1070916 Aurora Kinase inhibitor
Cat.No.S2740
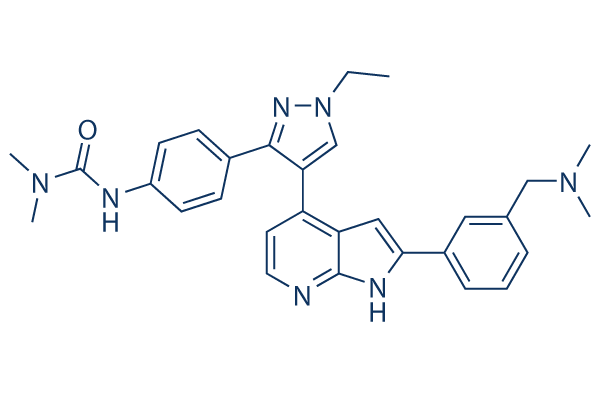
Chemical Structure
Molecular Weight: 507.63
Jump to
Quality Control
Batch:
Purity:
99.79%
99.79
| Related Targets | CDK HSP K-Ras PD-1/PD-L1 ROCK Wee1 DNA/RNA Synthesis Microtubule Associated Ras Casein Kinase |
|---|---|
| Other Aurora Kinase Inhibitors | Alisertib (MLN8237) Hesperadin Barasertib-HQPA (AZD2811) Tozasertib (VX-680) ZM 447439 MLN8054 Danusertib (PHA-739358) MK-5108 TCS7010 (Aurora A Inhibitor I) AMG-900 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| A549 cells | Proliferation assay | 6-7 d | Antiproliferative activity against human A549 cells after 6 to 7 days by celltiter-glo luminescence assay in absence of 70% human serum albumin, EC50=0.007 μM | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 102 mg/mL
(200.93 mM)
Ethanol : 102 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 507.63 | Formula | C30H33N7O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 942918-07-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCN1C=C(C(=N1)C2=CC=C(C=C2)NC(=O)N(C)C)C3=C4C=C(NC4=NC=C3)C5=CC=CC(=C5)CN(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Aurora B-INCENP
(Cell-free assay) 3.5 nM
Aurora C-INCENP
(Cell-free assay) 6.5 nM
FLT1
(Cell-free assay) 42 nM
Tie-2
(Cell-free assay) 59 nM
SIK
(Cell-free assay) 70 nM
FLT4
(Cell-free assay) 74 nM
FGFR1
(Cell-free assay) 76 nM
|
|---|---|
| In vitro |
GSK1070916 selectively inhibits Aurora B and Aurora C with Ki of 0.38 nM and 1.5 nM over Aurora A with Ki of 490 nM. Inhibition of Aurora B and Aurora C by this compound is time-dependent, with an enzyme-inhibitor dissociation half-life of >480 min and 270 min respectively. In addition, it is also a competitive inhibitor with respect to ATP. Human tumor cells treated with this inhibitor shows dose-dependent inhibition of phosphorylation on serine 10 of Histone H3, a substrate specific for Aurora B. Moreover, it inhibits the proliferation of tumor cells with EC50 values of <10 nM in over 100 cell lines spanning a broad range of tumor types, with a median EC50 of 8 nM. Although this compound has potent activity against proliferating cells, a dramatic shift in potency is observed in primary, nondividing, normal human vein endothelial cells. Furthermore, cells treated with this agent do not arrest in mitosis but instead fails to divide and become polyploid, ultimately leading to apoptosis. In another study, it is also reported high chromosome number associated with resistance to the inhibition of Aurora B and C suggests cells with a mechanism to bypass the high ploidy checkpoint are resistant to this chemical.
|
| Kinase Assay |
Kinase Assay
|
|
The ability of GSK1070916 to inhibit the Aurora enzymes is measured using in vivo kinase assays. The assays measure the ability of Aurora A, Aurora B and Aurora C to phosphorylate a synthetic peptide substrate. Biotin-Ahx-RARRRLSFFFFAKKK-NH2 is used for the Aurora A–TPX2 LEADseekerTM assay and 5FAM-PKAtide is used for the IMAPTM assay for all three Aurora kinases. To take into account time-dependent inhibition of Aurora enzymes, Aurora A–TPX2, Aurora B–INCENP and Aurora C–INCENP are incubated with this compound at various concentrations for 30 min before the reactions are initiated with the addition of substrates. For the Aurora A LEADseekerTM assay, final assay conditions are 0.5 nM Aurora A–TPX2, 1 μM peptide substrate, 6 mM MgCl2, 1.5 μM ATP, 0.003 μCi/μL [γ-33P] ATP in 50 mM Hepes, pH 7.2, 0.15 mg/mL BSA, 0.01% Tween-20, 5 mM DTT and 25 mM KCl. The reactions are incubated at room temperature (25 °C) for 120 min and terminated by the addition of LEADseekerTM beads in PBS containing EDTA (final concentration 2 mg/mL beads and 25 mM EDTA). The plates are then sealed, and the beads are allowed to settle overnight. Product formation is quantified using a Viewlux Imager. For the IMAPTM assays, Aurora A–TPX2 (final concentration 1 nM), Aurora B–INCENP (final concentration 2 nM) or Aurora C–INCENP (final concentration 2.5 nM) is added to the compound-containing plates in 5 μL of buffer (25 mM Hepes, pH 7.2, for Aurora A, 25 mM Hepes, pH 7.5, for Aurora B and 20 mM Hepes, pH 7.2, for Aurora C) containing 0.15 mg/mL BSA, 0.01% Tween 20 and 25 mM NaCl. This mixture is incubated at room temperature for 30 min. To start the reaction, 5 μL of a substrate solution is added containing the same Hepes buffer as used for the pre-incubation, 25 mM NaCl, MgCl2 (2, 4 and 4 mM for Aurora A, B and C respectively), DTT (4, 4 and 2 mM for Aurora A, B and C respectively), ATP (4, 4 and 10 μM for Aurora A, B and C respectively), 200 nM 5FAM-PKAtide, 0.01% Tween 20 and 0.15 mg/mL BSA. The reactions are incubated at room temperature for 120 min for Aurora A and B and 60 min for Aurora C. These reactions are then terminated by the addition of 10 μL of 1:500 (1:600 for Aurora C) Progressive Binding Reagent in 95% Progressive Binding Buffer A and 5% Progressive Binding Buffer B. Plates are incubated at room temperature for approx. 90–120 min (time allowed for equilibrium to be reached). Plates are read in a Molecular Devices Analyst plate reader in fluorescence polarization mode.
|
|
| In vivo |
GSK1070916 (25, 50, or 100 mg/kg) shows dose-dependent inhibition of phosphorylation of an Aurora B–specific substrate in mice and consistent with its broad cellular activity, this compound has antitumor effects in 10 human tumor xenograft models including breast, colon, lung, and two leukemia models.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































