research use only
Flavoxate HCl AChR antagonist
Cat.No.S4027
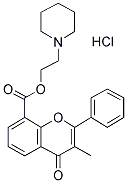
Chemical Structure
Molecular Weight: 427.92
Quality Control
Solubility
|
In vitro |
Water : 10 mg/mL
DMSO
: 3 mg/mL
(7.01 mM)
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 427.92 | Formula | C24H25NO4.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 3717-88-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | NSC-114649 | Smiles | CC1=C(OC2=C(C1=O)C=CC=C2C(=O)OCCN3CCCCC3)C4=CC=CC=C4.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
mAChR
12.2 μM
|
|---|---|
| In vitro |
Flavoxate displaces [3H]nitrendipine on the Ca2+ channels binding sites with IC50 of 254 μM. Flavoxate (>10 μM) suppresses carbachol-induced contractions in isolated rat detrusor strips with pD value of 4.55. Flavoxate (>10 μM) suppresses Ca2+-induced contractions in isolated rat detrusor strips with pIC50 value of 4.92. Flavoxate (0.01 μM −10 μM) inhibits CAMP formation in a concentration-dependent manner in membranes from the rat striatum and cerebral cortex, an action which is completely abolished by pretreating the membranes with pertussis toxin (PTX). Flavoxate causes a concentration-dependent reduction of the K+-induced contraction of human urinary bladder. Flavoxate inhibits the peak amplitude of voltage-dependent nifedipine-sensitive inward Ba2+ currents in a voltage- and concentration-dependent manner with Ki value of 10 μM in human detrusor myocytes. Flavoxate inhibits voltage-dependent nifedipine-sensitive inward Ba2+ currents in human detrusor myocytes at both 30 degrees C (Ki = 5.1 mM) and 37 degrees C (Ki = 4.6 mM).
|
| In vivo |
Flavoxate (10mg/kg) suppresses both the an initial, rapidly rising phasic contraction (phase 1) and the tonic contraction (phase 2) contractions to the same extent in rats. Flavoxate (10mg/kg) abolishes the bladder contractions without causing any change in the amplitude of the contractions in rats. Flavoxate (3 mg/kg) abolishes the efferent neural activity and the associated bladder contractions for about 10 minutes without changing the baseline vesical pressure in rats. ICV-injected (50 to 200 μg/rat) or IT-injected (100 to 200 μg/rat) Flavoxate abolishes rhythmic bladder contractions during and after injection for five to 15 minutes in a dose-dependent manner in rats. Flavoxate (3 mg/kg, i.v.) abolishes rhythmic bladder contractions and the maximal intervals of voiding contractions is 7.20 min.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































