- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Doramapimod (BIRB 796)
Home MAPK p38 MAPK inhibitor - JNK inhibitor - Raf inhibitor - Src inhibitor Doramapimod (BIRB 796)
For research use only.
Doramapimod (BIRB 796) is a pan-p38 MAPK inhibitor with IC50 of 38 nM, 65 nM, 200 nM and 520 nM for p38α/β/γ/δ in cell-free assays, and binds p38α with Kd of 0.1 nM in THP-1 cells, 330-fold greater selectivity versus JNK2, weak inhibition for c-RAF, Fyn and Lck, insignificant inhibition of ERK-1, SYK, IKK2.
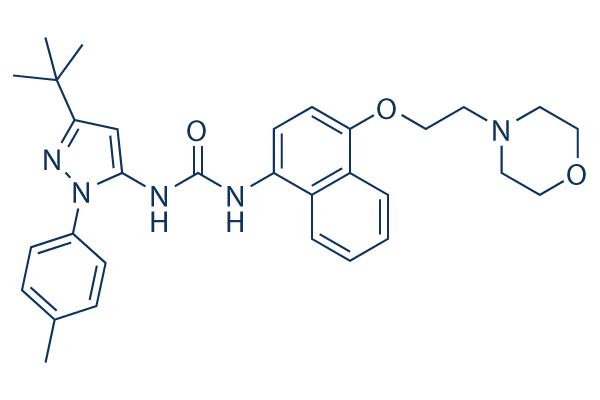
Doramapimod (BIRB 796) Chemical Structure
CAS No. 285983-48-4
Purity & Quality Control
Batch:
Purity:
99.99%
99.99
Doramapimod (BIRB 796) Related Products
| Related Targets | p38α p38β | Click to Expand |
|---|---|---|
| Related Products | Adezmapimod (SB203580) SB202190 Ralimetinib (LY2228820) dimesylate PH-797804 VX-702 Losmapimod SB239063 Neflamapimod (VX-745) BMS-582949 Skepinone-L Asiatic Acid TAK-715 Pamapimod Pexmetinib SD 0006 PD 169316 R1487 | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library MAPK Inhibitor Library Cell Cycle compound library TGF-beta/Smad compound library Anti-alzheimer Disease Compound Library | Click to Expand |
Signaling Pathway
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HOP-92 | Growth Inhibition Assay | IC50=24.3838 μM | SANGER | |||
| NCI-H2228 | Growth Inhibition Assay | IC50=23.6668 μM | SANGER | |||
| CAPAN-1 | Growth Inhibition Assay | IC50=22.1884 μM | SANGER | |||
| SK-MEL-1 | Growth Inhibition Assay | IC50=20.3683 μM | SANGER | |||
| DB | Growth Inhibition Assay | IC50=18.7923 μM | SANGER | |||
| AN3-CA | Growth Inhibition Assay | IC50=18.1 μM | SANGER | |||
| EW-13 | Growth Inhibition Assay | IC50=17.9516 μM | SANGER | |||
| LC-2-ad | Growth Inhibition Assay | IC50=17.4366 μM | SANGER | |||
| ES8 | Growth Inhibition Assay | IC50=17.167 μM | SANGER | |||
| NCI-H1581 | Growth Inhibition Assay | IC50=17.0447 μM | SANGER | |||
| HMV-II | Growth Inhibition Assay | IC50=14.2309 μM | SANGER | |||
| BEN | Growth Inhibition Assay | IC50=13.1264 μM | SANGER | |||
| NBsusSR | Growth Inhibition Assay | IC50=10.8235 μM | SANGER | |||
| MS-1 | Growth Inhibition Assay | IC50=10.8235 μM | SANGER | |||
| BFTC-905 | Growth Inhibition Assay | IC50=10.1233 μM | SANGER | |||
| NCI-SNU-1 | Growth Inhibition Assay | IC50=9.06739 μM | SANGER | |||
| MPP-89 | Growth Inhibition Assay | IC50=8.46251 μM | SANGER | |||
| T-24 | Growth Inhibition Assay | IC50=8.40673 μM | SANGER | |||
| KP-N-YN | Growth Inhibition Assay | IC50=8.2019 μM | SANGER | |||
| IST-MES1 | Growth Inhibition Assay | IC50=7.95637 μM | SANGER | |||
| NCI-H358 | Growth Inhibition Assay | IC50=7.538 μM | SANGER | |||
| GOTO | Growth Inhibition Assay | IC50=6.39161 μM | SANGER | |||
| DU-145 | Growth Inhibition Assay | IC50=3.93811 μM | SANGER | |||
| EoL-1-cell | Growth Inhibition Assay | IC50=0.34763 μM | SANGER | |||
| KYSE-270 | Growth Inhibition Assay | IC50=24.5573 μM | SANGER | |||
| HCC1806 | Growth Inhibition Assay | IC50=24.7799 μM | SANGER | |||
| HuO-3N1 | Growth Inhibition Assay | IC50=25.8185 μM | SANGER | |||
| HOS | Growth Inhibition Assay | IC50=25.9292 μM | SANGER | |||
| KYSE-510 | Growth Inhibition Assay | IC50=26.1612 μM | SANGER | |||
| COLO-741 | Growth Inhibition Assay | IC50=26.3329 μM | SANGER | |||
| H-EMC-SS | Growth Inhibition Assay | IC50=26.9245 μM | SANGER | |||
| HCC1937 | Growth Inhibition Assay | IC50=27.2238 μM | SANGER | |||
| NCI-H2126 | Growth Inhibition Assay | IC50=27.3975 μM | SANGER | |||
| NCI-H1703 | Growth Inhibition Assay | IC50=28.0413 μM | SANGER | |||
| U-2-OS | Growth Inhibition Assay | IC50=28.5515 μM | SANGER | |||
| DBTRG-05MG | Growth Inhibition Assay | IC50=28.5651 μM | SANGER | |||
| MHH-ES-1 | Growth Inhibition Assay | IC50=31.941 μM | SANGER | |||
| HCC1419 | Growth Inhibition Assay | IC50=32.1119 μM | SANGER | |||
| HOP-62 | Growth Inhibition Assay | IC50=32.2701 μM | SANGER | |||
| AM-38 | Growth Inhibition Assay | IC50=32.9931 μM | SANGER | |||
| NCI-H2009 | Growth Inhibition Assay | IC50=33.4007 μM | SANGER | |||
| EM-2 | Growth Inhibition Assay | IC50=33.5511 μM | SANGER | |||
| SW1116 | Growth Inhibition Assay | IC50=34.4838 μM | SANGER | |||
| SK-N-AS | Growth Inhibition Assay | IC50=35.0714 μM | SANGER | |||
| ChaGo-K-1 | Growth Inhibition Assay | IC50=35.6032 μM | SANGER | |||
| RT-112 | Growth Inhibition Assay | IC50=35.9879 μM | SANGER | |||
| HTC-C3 | Growth Inhibition Assay | IC50=36.2355 μM | SANGER | |||
| SK-NEP-1 | Growth Inhibition Assay | IC50=36.6106 μM | SANGER | |||
| LB831-BLC | Growth Inhibition Assay | IC50=37.6541 μM | SANGER | |||
| CTB-1 | Growth Inhibition Assay | IC50=38.4512 μM | SANGER | |||
| MOLT-4 | Growth Inhibition Assay | IC50=38.8391 μM | SANGER | |||
| SW756 | Growth Inhibition Assay | IC50=40.9385 μM | SANGER | |||
| CAL-72 | Growth Inhibition Assay | IC50=42.03 μM | SANGER | |||
| KNS-62 | Growth Inhibition Assay | IC50=42.6296 μM | SANGER | |||
| KARPAS-299 | Growth Inhibition Assay | IC50=43.3233 μM | SANGER | |||
| HEL | Growth Inhibition Assay | IC50=45.4646 μM | SANGER | |||
| KP-4 | Growth Inhibition Assay | IC50=46.7361 μM | SANGER | |||
| NEC8 | Growth Inhibition Assay | IC50=47.1661 μM | SANGER | |||
| G-402 | Growth Inhibition Assay | IC50=48.7012 μM | SANGER | |||
| Sf21 | Function assay | Binding affinity to wild type human biotin labelled p38 alpha (9 to 352 residues) expressed in sf21 insect cells SPR analysis, Kd = 0.000123 μM. | 28834431 | |||
| THP1 | Function assay | Inhibition of LPS-induced TNFalpha production in human THP1 cells, IC50 = 0.013 μM. | 18325768 | |||
| U937 | Antiinflammatory assay | 2 hrs | Antiinflammatory activity in differentiated human U937 cells assessed as inhibition of LPS-induced TNFalpha production preincubated for 2 hrs followed by LPS-stimulation for 4 hrs by sandwich ELISA relative to vehicle-treated control, IC50 = 0.015 μM. | 26800309 | ||
| THP1 | Function assay | Inhibition of LPS-induced TNFalpha production in human THP1 cells, IC50 = 0.018 μM. | 19356929 | |||
| THP1 | Function assay | Inhibition of LPS-induced tumor necrosis factor-alpha (TNF-alpha) production in THP-1 cells, EC50 = 0.018 μM. | 12086485 | |||
| THP | Function assay | Tested for inhibition of Tumor necrosis factor, alpha in THP cells, EC50 = 0.018 μM. | 14561087 | |||
| THP1 | Function assay | Inhibition of LPS-induced TNFalpha production in THP1 cells, IC50 = 0.018 μM. | 17560108 | |||
| THP1 | Function assay | Inhibition of LPS-stimulated TNFalpha production in human THP1 cells, IC50 = 0.018 μM. | 18462940 | |||
| HLF | Function assay | Inhibition of p38alpha phosphorylation in IL-1-alpha-stimulated HLF cells, IC50 = 0.047 μM. | 18602262 | |||
| HLF | Function assay | Inhibition of HSP27 phosphorylation in IL-1-alpha-stimulated HLF cells, IC50 = 0.058 μM. | 18602262 | |||
| HEK293F | Function assay | Inhibition of sodium arsenate activated N-terminal GST-tagged Brugia malayi MPK1 expressed in HEK293F cells using FAM-p38tide as substrate by IMAP assay, IC50 = 0.14 μM. | 29541362 | |||
| BL21(DE3) | Function assay | Inhibition of p38alpha active form expressed in Escherichia coli BL21(DE3) cells by HTRF assay, IC50 = 0.25 μM. | 19928858 | |||
| whole blood cells | Antiinflammatory assay | 4 hrs | Antiinflammatory activity in human whole blood cells assessed as inhibition of LPS-induced TNFalpha production after 4 hrs by time-resolved fluorescence assay, IC50 = 0.6 μM. | 22749282 | ||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Doramapimod (BIRB 796) is a pan-p38 MAPK inhibitor with IC50 of 38 nM, 65 nM, 200 nM and 520 nM for p38α/β/γ/δ in cell-free assays, and binds p38α with Kd of 0.1 nM in THP-1 cells, 330-fold greater selectivity versus JNK2, weak inhibition for c-RAF, Fyn and Lck, insignificant inhibition of ERK-1, SYK, IKK2. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | The first p38 MAPK inhibitor to be tested in a phase III clinical trial. | ||||||||||
| Targets |
|
| In vitro | ||||
| In vitro | BIRB 796 shows no significant inhibition to ERK-1, SYK, IKK2β, ZAP-70, EGF receptor kinase, HER2, protein kinase A (PKA), PKC, PKC-α, PKC-β (I and II) and PKC-γ. BIRB 796 greatly improves binding affinity by forming a hydrogen bond between the morpholine oxygen and the ATP-binding domain of p38α. BIRB 796 represents one of the most potent and slowest dissociating inhibitors against human p38 MAP kinase now known. [1] BIRB 796 potently inhibits c-Raf-1 and Jnk2α2 with IC50 of 1.4 and 0.1 nM, respectively. [2] BIRB796 also inhibits the activity and the activation of SAPK3/p38γ at a higher concentration than it does in p38α. BIRB796 blocks the stress-induced phosphorylation of the scaffold protein SAP97, which is a physiological substrate of SAPK3/p38γ. BIRB796 blocks JNK1/2 activation and activity in HEK293 cells, while not inhibits the activation and activity of ERK1/ERK2 in Hela cells. Moreover, the binding of BIRB796 to the p38 MAPKs or JNK1/2 is impairing their phosphorylation by the upstream kinase MKK6 or MKK4 rather than enhancing their dephosphorylation. [3] BIRB 796 blocks baseline and upregulation of p38 MAPK and Hsp27 phosphorylation, thereby enhancing cytotoxicity and caspase activation. BIRB 796 downregulates IL-6 and VEGF secretion in BMSCs triggered by TNF-α and TGF-β1. [4] BIRB-796 has a pyrazole scaffold that places a lipophilic t-butyl group into the lower selectivity site and a tolyl ring into the upper selectivity site. BIRB-796 also inhibits B-Raf and Abl with IC50 of 83 nM and 14.6 μM, respectively. [5] |
|||
|---|---|---|---|---|
| Kinase Assay | Procedures for the THP-1 cellular assay for inhibition of LPS-stimulated TNF-α production | |||
| THP-1 cells are preincubated in the presence and absence of BIRB 796 for 30 min. Cell mixture is stimulated with LPS (1 μg/mL final) and incubation continued overnight (18−24 hours) as above. Supernatant is analyzed for human TNF-α by a commercially available ELISA. Data are combined and analyzed by nonlinear regression using a three parameter logistic model to obtain an EC50 value. BIRB 796 is analyzed in each experiment and the 95% confidence intervals for the EC50 are between 16 and 22 nM. | ||||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p-p38 / γ-H2AX mTOR / p-S6K / S6K / p-MK2 / MK2 / p-Hsp27 / Hsp27 p-p38 / p38 |
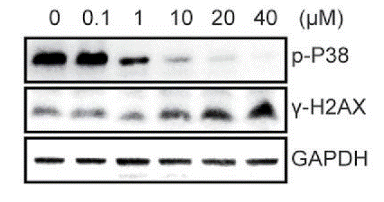
|
27082306 | |
| In Vivo | ||
| In vivo | BIRB 796 (30 mg/kg) inhibits 84% of TNF-α in LPS-stimulated mice and demonstrates efficacy in a mouse model of established collagen-induced arthritis. [1] BIRB 796 has good pharmacokinetic performance even after oral administration in mice. [2] |
|
|---|---|---|
| Animal Research | Animal Models | Collagen-induced arthritis in female Balb/c mice |
| Dosages | 1 mg/kg (intravenous) or 10 mg/kg (oral) | |
| Administration | Intravenous injection or by oral | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02211885 | Completed | Healthy |
Boehringer Ingelheim |
October 2002 | Phase 1 |
|
Chemical Information & Solubility
| Molecular Weight | 527.66 | Formula | C31H37N5O3 |
| CAS No. | 285983-48-4 | SDF | Download Doramapimod (BIRB 796) SDF |
| Smiles | CC1=CC=C(C=C1)N2C(=CC(=N2)C(C)(C)C)NC(=O)NC3=CC=C(C4=CC=CC=C43)OCCN5CCOCC5 | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 100 mg/mL ( (189.51 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 100 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field