- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
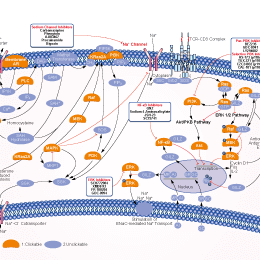
Procaine HCl Sodium Channel inhibitor
Procaine (Novocaine) is an inhibitor of sodium channel, NMDA receptor and nAChR with IC50 of 60 μM, 0.296 mM and 45.5 μM, which is also an inhibitor of 5-HT3 with KD of 1.7 μM.
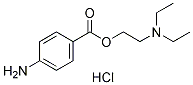
Chemical Structure
Molecular Weight: 272.77
Purity & Quality Control
Related Products
| Related Products | Camostat Mesilate EIPA A-803467 cariporide Veratramine Bulleyaconi cine A Vinpocetine | Click to Expand |
|---|---|---|
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Ion Channel Ligand Library Exosome Secretion Related Compound Library Calcium Channel Blocker Library | Click to Expand |
Signaling Pathway
Mechanism of Action
| Targets |
|
|---|
In vitro |
||||
| In vitro | Procaine acts mainly by inhibiting sodium influx through voltage gated sodium channels in the neuronal cell membrane of peripheral nerves. When the influx of sodium is interrupted, an action potential cannot arise and signal conduction is thus inhibited. The receptor site is thought to be located at the cytoplasmic (inner) portion of the sodium channel. [1] Procaine has also been shown to bind or antagonize the function of N-methyl-D-aspartate (NMDA) receptors [2] as well as nicotinic acetylcholine receptors [3] and the serotonin receptor-ion channel complex. [4] Procaine is an inhibitor of the mechanisms of Ca-induced Ca release and caffeine-induced Ca release in various types of muscle preparations. 0.5 mM Procaine blocks individual sarcoplasmic reticulum Ca2+ release channels in planarlipid bilayers. Procaine does not reduce the single channel conductance nor appreciably shorts the mean open times of the channel, rather, it increases the longest closed time. [5] Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. 0.5 mM Procaine produces a 40% reduction in 5-methylcytosine DNA in MCF-7 breast cancer cell line. Procaine can also bind to CpG-enriched DNA, and demethylates densely hypermethylated CpG islands, leading to restoring gene expression of epigenetically silenced genes. Procaine treatment (0.5 mM) induces an increase in the mitotic index of cells in M phase. Procaine treatment (1 mM) reduces cell proliferation by ~40%. [6] Procaine influences red cell shape and deformability. 45 mM Procaine almost completely prevents the discocyte-echinocyte transformation associated with ATP depletion. Similar concentrations of Procaine normalize the viscosity and filterability, but have no effect on cell volume, osmotic fragility, or monovalent cation composition of cells undergoing ATP depletion. [7] | |||
|---|---|---|---|---|
In Vivo |
||
| In vivo | Procaine is an excitant of limbic system cells. 15 mg/kg Procaine increases cellular activity in amygdala ventral hippocampus, nucleus accumbens, temporal neocortex and ventromedial hypothalamus of awaken cat. Procaine facilitates transmission of evoked excitatory activity from the amygdala to the ventromedial hypothalamus. [8] Procaine influences frequency and amplitude of reticularly elicited hippocampal rhythmical slow activity. Procaine (0.5 μL, 20% wt/vol) injected at points in the ascending system anterior to the supramamillary nucleus, in the region of the medial forebrain bundle or in the medial septum, reduces the amplitude of reticularly elicited rhythmical slow activity (RSA) but has no effect on its frequency. Procaine injected at points in the ascending system from just anterior to the reticular formation stimulation site, up to, and including the supramamillary nucleus, reduces both the frequency and amplitude of reticularly elicited RSA. [9] Procaine (80mg/kg) increases the duration and propagation of epileptiform afterdischarges (ADs) produced by electrical stimulation of the amygdala in rats. Porcaine also increases the rate of seizure development (kindling) produced by repeated stimulation of the amygdala. Procaine would itself act as convulsants in well kindled subjects. Procaine produces a weak but significant increase in the amplitude of the transcallosal evoked potential. [10] Procaine influences generation of auditory brain stem responses (ABRs). Procaine (30 μL of 1% solution) injection into the trapezoid body of guinea pig affects many of the components of the scalp-derived ABR: N2 is delayed making P2 broader in duration, P3 and N3 are lost, P4 is shortened in latency, broadened in duration but unaffected in amplitude, and N4 is considerably attenuated. Only P1 and N1 are unaffected by the procaine injection. [11] Procaine increases the therapeutic index of cisplatin by improving antitumor activity of cisplatin and reducing its nephrotoxicity. Simultaneous administration of cisplatin and Procaine (40 mg/kg) to BDF1 mice produces 50% lethal dose (LD50) and 90% lethal dose (LD90) values approximately two times higher than those observed with cisplatin alone. Simultaneous administration produces a higher cure rates compared with cisplatin alone (50% vs 9%). The increased blood urea nitrogen (BUN) levels observed 4-7 days following a single administration of cisplatin, as well as the tubular degenerative changes detected by light microscopy, are not observed when the same doses of cisplatin are given simultaneously with Procaine. [12] | |
|---|---|---|
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03805503 | Completed | Spinal Anesthesia |
University Hospital Ghent |
September 16 2015 | Phase 4 |
| NCT02287870 | Completed | Anesthesia |
Jinling Hospital China |
January 2008 | Phase 4 |
References |
|
Chemical Information
| Molecular Weight | 272.77 | Formula | C13H20N2O2.HCl |
| CAS No. | 51-05-8 | SDF | Download SDF |
| Synonyms | Novocaine HCl | ||
| Smiles | CCN(CC)CCOC(=O)C1=CC=C(C=C1)N.Cl | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 55 mg/mL ( (201.63 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : 55 mg/mL Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field