- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Lamivudine Reverse Transcriptase inhibitor
Cat.No.S1706
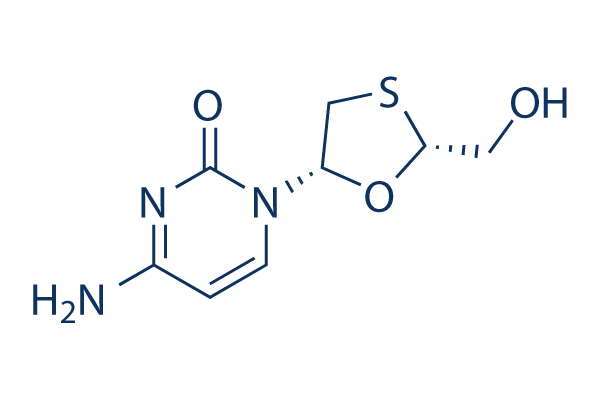
Chemical Structure
Molecular Weight: 229.26
Jump to
Quality Control
Batch:
Purity:
99.94%
99.94
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite HIV HCV Protease |
|---|---|
| Other Reverse Transcriptase Products | Dapivirine (TMC120) Salicylanilide Fangchinoline Bifendate 3'-Fluoro-3'-deoxythymidine (Alovudine) Ulonivirine Lersivirine (UK-453061) 4-Chloro-2-(trifluoroacetyl)aniline hydrochloride |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MT-4 | Function assay | Concentration required to inhibit syntica formation by 50% in HIV-1 infected MT-4 cells, IC50=0.1μM | 8035429 | |||
| PBMC | Function assay | Concentration required for antiviral activity in PBMC (human peripheral blood mononuclear cells) infected with HIV-1 strain LAV, EC50=0.0018μM | 8410975 | |||
| PBMC | Function assay | Concentration required for antiviral activity in PBMC (human peripheral blood mononuclear cells) infected with HIV-1 strain LAV, EC50=0.21μM | 8410975 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=0.0018μM | 8423591 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=0.06μM | 8423591 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=0.21μM | 8423591 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=10.1μM | 8423591 | |||
| 2.2.15 | Function assay | In vitro effective concentration against hepatitis B virus was determined in 2.2.15 cells, EC50=0.008μM | 9301659 | |||
| PBMC | Anti-HIV-1 assay | In vitro anti-HIV activity in peripheral blood mononuclear cells, EC50=0.07μM | 9301659 | |||
| 2.2.15 | Antiviral assay | Antiviral activity against Hepatitis B virus (HBV) in 2.2.15 cells, EC50=0.008μM | 11052795 | |||
| PBMC | Antiviral assay | Antiviral activity against Immunodeficiency virus type-1 (HIV-1) in peripheral blood mononuclear cells, EC50=0.07μM | 11052795 | |||
| H9 | Antiviral assay | Antiviral activity against HIV1 3B in human H9 cells assessed as inhibition of virus-induced cytopathic effect by formazan-based conventional colorimetric technique, EC50=0.06μM | 11430019 | |||
| H9 | Antiviral assay | Antiviral activity against HIV1 RF in human H9 cells assessed as inhibition of virus-induced cytopathic effect by formazan-based conventional colorimetric technique, EC50=0.06μM | 11430019 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV-1xxBRU mutant strain in human PBM cells, EC90=0.08μM | 12383014 | |||
| PBMC | Function assay | Activity against Lamivudine-resistant virus (HIV-1XXBRU) in human PBM cells, expressed as EC50, EC50=0.035μM | 12852755 | |||
| PBMC | Function assay | In vitro concentration required to inhibit the HIV-1 LA1 replication on PBMCs cell (peripheral blood mononuclear cells), EC50=0.01μM | 12852943 | |||
| PBMC | Function assay | Effective concentration to inhibit HIV-1 LAI cytopathicity in PBMC cells was determined in vitro, EC50=0.0116μM | 14971898 | |||
| HepG2.2.15 | Function assay | Effective concentration to inhibit hepatitis B virus cytopathicity in HepG2.2.15 cells was determined in vitro, EC50=0.2μM | 14971898 | |||
| MT-4 | Function assay | Effective concentration to inhibit HIV-1 IIIB cytopathicity in MT-4 cells was determined in vitro, EC50=0.5μM | 14971898 | |||
| PBMC | Antiviral assay | Antiviral activity against wild type HIV virus(xxBRU) in Human peripheral blood mononuclear (PBM) cells, EC90=0.08μM | 15027854 | |||
| PBMC | Function assay | Effective concentration against Wild Type HIV-1 virus in human peripheral blood mononuclear cells, EC50=0.041μM | 15189036 | |||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against HIV1 3B infected in human CEM-SS cells assessed as inhibition of virus-induced cytopathic effect after 6 days by MTS assay, ED50=0.36μM | 15217281 | ||
| HOG.R5 | Antiviral assay | 4 days | Antiviral activity against HIV1 3B infected in human HOG.R5 cells after 4 days, IC50=1.2μM | 15217281 | ||
| 2.2.15 | Function assay | Inhibition of HBV replication in 2.2.15 cells by DNA hybridization assay, EC50=0.02μM | 15615545 | |||
| 2.2.15 | Function assay | Effective concentration required to inhibit hepatitis B virus replication in 2.2.15 cells in DNA hybridization assay, EC50=0.05μM | 15634003 | |||
| PBMC | Function assay | In vitro effective concentration against Lamivudine resistant wild type HIV-1 in human peripheral blood mononuclear cells, EC50=0.027μM | 15916425 | |||
| PBMC | Function assay | In vitro effective concentration against Lamivudine resistant wild type HIV-1 in human peripheral blood mononuclear cells, EC90=0.25μM | 15916425 | |||
| 2.2.15 | Function assay | Effective concentrations at which 2-fold depression of extracellular virion DNA (HBV) was observed in 2.2.15 Cells, EC50=0.06μM | 16033283 | |||
| 2.2.15 | Function assay | Effective concentrations at which 2-fold depression of extracellular virion DNA (HBV) was observed in 2.2.15 Cells, EC90=0.17μM | 16033283 | |||
| 2.2.15 | Function assay | Effective concentrations at which 10-fold depression of intracellular intermediates of HBV DNA was observed in 2.2.15 Cells, EC50=0.23μM | 16033283 | |||
| 2.2.15 | Function assay | Effective concentrations at which 10-fold depression of intracellular intermediates of HBV DNA was observed in 2.2.15 Cells, EC90=0.69μM | 16033283 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of extracellular virion DNA release in cultured human hepatoblastoma 2.2.15 cells, EC50=0.048μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of extracellular virion DNA release in cultured human hepatoblastoma 2.2.15 cells, EC90=0.151μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of intracellular HBV DNA replicative intermediates in cultured human hepatoblastoma 2.2.15 cells, EC50=0.251μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of intracellular HBV DNA replicative intermediates in cultured human hepatoblastoma 2.2.15 cells, EC90=0.648μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of wild type HBV transfected in human hepatoblastoma 2.2.15 cells, EC50=2.1μM | 16539393 | |||
| hepatoma 2.2.15 | Antiviral assay | Antiviral activity against wild type HBV in human hepatoma 2.2.15 cells, EC50=2.1μM | 16759112 | |||
| 2.2.15 | Function assay | Inhibition of HBV replication in 2.2.15 cells by DNA hybridization assay, EC50=0.02μM | 17004726 | |||
| MDCK2 | Function assay | 10 uM | Inhibition of human MRP2 expressed in MDCK2 cells assessed as increase in intracellular CMF fluorescence at 10 uM by CMFDA assay | 17172311 | ||
| MDCK2 | Function assay | 10 uM | Inhibition of human MRP3 expressed in MDCK2 cells assessed as increase in intracellular CMF fluorescence at 10 uM by CMFDA assay | 17172311 | ||
| MDCK2 | Function assay | 10 uM | Inhibition of human MRP1 expressed in MDCK2 cells assessed as increase in intracellular CMF fluorescence at 10 uM by CMFDA assay | 17172311 | ||
| Huh7 | Function assay | Inhibition of wild type HBV replication in Huh7 cells, EC50=1μM | 17371827 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV1 LAI in human PBM cells, EC50=0.026μM | 17403996 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV1 LAI in human PBM cells, EC90=0.12μM | 17403996 | |||
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV in HepG2.2.15 cells assessed as decrease in extracellular viral DNA level by RT-PCR, EC50=0.1μM | 17404006 | |||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBV genotype D with reverse transcriptase L80I mutation in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.03μM | 17438047 | ||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBeAg-deficient HBV genotype D with precore G1896A mutation and reverse transcriptase L80I mutation in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.03μM | 17438047 | ||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBV genotype D in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.41μM | 17438047 | ||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBeAg-deficient HBV genotype D with precore G1896A mutation in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.46μM | 17438047 | ||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against HBV in ayw wild type HepG2(2.2.15) cells assessed as inhibition of viral DNA replication, IC50=0.05μM | 17488817 | |||
| HepG2(2.2.15) | Function assay | Inhibition of HBV promoter in human HepG2(2.2.15) cells by luciferase reporter gene assay, IC50=0.08μM | 17488817 | |||
| HepW10 | Antiviral assay | Antiviral activity against HBV in adriamycin-resistant wild type HepW10 cells assessed as inhibition of viral DNA replication, IC50=0.1μM | 17488817 | |||
| HBV-Met | Antiviral assay | Antiviral activity against HBV in mouse ayw wild type HBV-Met cells assessed as inhibition of viral DNA replication, IC50=0.2μM | 17488817 | |||
| HepG2.2.15 | Function assay | 8 days | Inhibition of cytoplasmic HBV DNA synthesis in HepG2.2.15 cells after 8 days, IC50=0.38μM | 17499889 | ||
| PBMC | Function assay | 300 mg | 7 days | Cmax in HIV-infected human assessed as Lamivudine monophosphate level in PBMC at 300 mg QD for 7 days measured per 10'6 cells in presence of zidovudine, Cmax=0.0000008μM | 17664328 | |
| PBMC | Function assay | 300 mg | 7 days | Cmax in HIV-infected human assessed as Lamivudine diphosphate level in PBMC at 300 mg QD for 7 days measured per 10'6 cells in presence of zidovudine, Cmax=0.0000044μM | 17664328 | |
| PBMC | Function assay | 300 mg | 7 days | Cmax in HIV-infected human assessed as Lamivudine triphosphate level in PBMC at 300 mg QD for 7 days measured per 10'6 cells in presence of zidovudine, Cmax=0.0000048μM | 17664328 | |
| MAGIC-5A | Antiviral assay | 40 hrs | Antiviral activity against wild type HIV1 NL4-3 produced from full length pR9deltaApa infected in human MAGIC-5A cells assessed as inhibition of Lac+ foci expression after 40 hrs, EC50=0.88μM | 17967913 | ||
| MAGIC-5A | Antiviral assay | 40 hrs | Antiviral activity against wild type HIV2 ROD produced from full length pROD9 infected in human MAGIC-5A cells assessed as inhibition of Lac+ foci expression after 40 hrs, EC50=2μM | 17967913 | ||
| MAGIC-5A | Antiviral assay | 40 hrs | Antiviral activity against multinucleoside-resistant HIV1 NL4-3 harboring A62V, V75I, F77L, F116Y, Q151M mutation in viral reverse transcriptase infected in human MAGIC-5A cells assessed as inhibition of Lac+ foci expression after 40 hrs, EC50=3.7μM | 17967913 | ||
| PBMC | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-ASM 044 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.04μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.04μM | 18316521 | ||
| MT2 | Antiviral assay | Antiviral activity against 0.005 MOI HIV1 NL4-3 harboring wild type RT infected in human MT2 cells, EC50=0.04μM | 18316521 | |||
| MT2 | Antiviral assay | Antiviral activity against HIV1 subtype B-3B infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.047μM | 18316521 | |||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.063μM | 18316521 | ||
| PBMC | Antiviral assay | Antiviral activity against HIV1 subtype B-ASM 034 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.071μM | 18316521 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV1 subtype B-92US076 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.081μM | 18316521 | |||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.086μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-RF infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.105μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-NL4-3 infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.171μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-LAI infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.182μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-SF-2 infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.183μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-HXB2 infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.202μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.219μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.372μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=0.462μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.554μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=0.667μM | 18316521 | ||
| PBMC | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-93US143 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.85μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.927μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.969μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against pseudotype HIV1 NL-RLuc infected in human MT2 cells measured on day 5 postinfection by luciferase reporter gene assay, EC50=1.14μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=1.259μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=1.683μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=1.985μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=2.445μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=2.47μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=3.247μM | 18316521 | ||
| HepG2(2.2.15) | Antiviral assay | 24 hrs | Antiviral activity against HBV in human HepG2(2.2.15) cells assessed as extracellular HBV virion DNA level after 24 hrs, EC50=0.048μM | 19072694 | ||
| HepG2(2.2.15) | Antiviral assay | 24 hrs | Antiviral activity against HBV in human HepG2(2.2.15) cells assessed as replication intermediates after 24 hrs, EC50=0.15μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against lamivudine-resistant wild type HBV in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=0.2μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against lamivudine-resistant HBV reverse transcriptase 236T mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=0.3μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant wild type HBV in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.3μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase M204V mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.5μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase L180M mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.6μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase LM/reverse transcriptase MV double mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.6μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase M204I mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.8μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase 236T mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=7.7μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against lamivudine-resistant HBV reverse transcriptase L180M mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=10μM | 19072694 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against wild type HBV infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC50=0.2μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase N236T mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC50=0.3μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase N236T mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC90=1.5μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against wild type HBV infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC90=3μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase L180M mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC50=16μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase L180M mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC90=41μM | 19398648 | ||
| MT2 | Antiviral assay | Antiviral activity against HIV1 infected in human MT2 cells assessed as inhibition of viral replication, IC50=0.6μM | 19596885 | |||
| HeLa P4/R5 | Antiviral assay | Antiviral activity against HIV1 infected in human HeLa P4/R5 cells assessed as inhibition of viral replication, IC50=0.78μM | 19596885 | |||
| TZM-bl | Antiviral assay | 72 hrs | Antiviral activity against HIV1 pNL4-3 harboring Reverse transcriptase P119S mutant gene infected in TZM-bl cells after 72 hrs by luciferase assay, EC50=0.47μM | 19704131 | ||
| TZM-bl | Antiviral assay | Antiviral activity against wild-type HIV1 pNL4-3 infected in TZM-bl cells by luciferase assay, EC50=0.65μM | 19704131 | |||
| TZM-bl | Antiviral assay | 72 hrs | Antiviral activity against HIV1 pNL4-3 harboring Reverse transcriptase T165A mutant gene infected in TZM-bl cells after 72 hrs by luciferase assay, EC50=1.08μM | 19704131 | ||
| TZM-bl | Antiviral assay | 72 hrs | Antiviral activity against HIV1 pNL4-3 harboring Reverse transcriptase P119S/T165A mutant gene infected in TZM-bl cells after 72 hrs by luciferase assay, EC50=2.1μM | 19704131 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as inhibition of viral DNA replication, IC50=0.22μM | 19897363 | |||
| 1.3ES2 | Antiviral assay | 6 days | Antiviral activity against Hepatitis B virus ayw in human 1.3ES2 cells stably transfected with 1.3 fold wild type HBV ayw strain genome cells assessed as reduction of viral DNA level after 6 days by RT-PCR | 20061158 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against wild type HBV infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC50=0.2μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against ADV-resistant HBV N236T mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC50=0.2μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against wild type HBV infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC90=0.7μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against ADV-resistant HBV N236T mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC90=0.8μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against 3TC-resistant HBV with polymerase L180M mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC50=5.3μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against 3TC-resistant HBV with polymerase L180M mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC90=20μM | 20117930 | ||
| HepG2(2.2.15) | Antiviral assay | 13 uM | 72 hrs | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as decrease in intracellular viral DNA level at 13 uM after 72 hrs by RT-PCR analysis | 20176893 | |
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype D isolate 8 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=3.25μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype A isolate 1 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=3.54μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype C isolate 6 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=3.9μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype C isolate 7 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.06μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype BF isolate 5 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.24μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype A isolate 2 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.48μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 NL4-3 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.68μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype B isolate 3 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.78μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype B isolate 4 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=5.43μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 expressing reverse transcriptase K103N/Y181C/G190A mutant infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=7.16μM | 20308377 | ||
| HepG2(2.2.15) | Antiviral assay | 8 days | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as inhibition of viral cytoplasmic DNA synthesis after 8 days by RT-PCR analysis, IC50=0.16μM | 20639110 | ||
| HepG2(2.2.15) | Cytotoxicity assay | 8 days | Cytotoxicity against human HepG2(2.2.15) cells after 8 days by MTT assay, CC50=5μM | 20639110 | ||
| MT2 | Antiviral assay | 2 to 3 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect measured after 2 to 3 days by PCR, EC50=0.8μM | 21115794 | ||
| MT2 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B harboring reverse transcriptase M184V, M184I mutant infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect selected on day 14 after 4 passages by PCR analysis, EC50=0.8μM | 21115794 | |||
| MT2 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B harboring reverse transcriptase M184V mutant infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect selected on day 14 after 4 passages by PCR analysis, EC50=0.8μM | 21115794 | |||
| MT2 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B harboring reverse transcriptase M184V, M184I, K82N mutant infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect selected on day 14 after 4 passages by PCR analysi, EC50=0.8μM | 21115794 | |||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as reduction in viral DNA by Southern blotting, EC50=0.06μM | 21333535 | |||
| Huh7 | Antiviral assay | 1 day | Antiviral activity against Hepatitis B virus infected in human Huh7 cells assessed as reduction in viral DNA after 1 day by Southern blotting, EC50=0.1μM | 21333535 | ||
| HuH7 | Cytotoxicity assay | 4 days | Cytotoxicity against human HuH7 cells after 4 days by neutral red dye uptake assay, CC50=0.1μM | 21333535 | ||
| HepG2(2.2.15) | Antiviral assay | 4 days | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as inhibition of viral DNA replication after 4 days by RT-PCR analysis, IC50=0.33μM | 21524588 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against wild type Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as inhibition of virus replication treated daily for 9 days by quantitative blot hybridization method, EC50=0.2μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against adefovir-resistant Hepatitis B virus harboring reverse transcriptase N236T mutant infected in human HepG2(2.2.15) cells assessed as inhibition of virus replication treated daily for 9 days by quantitative blot hybridization meth, EC50=0.2μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against wild type Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as reduction of infectious virus titer treated daily for 9 days by quantitative Southern blot hybridization assay, EC90=0.6μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against adefovir-resistant Hepatitis B virus harboring reverse transcriptase N236T mutant infected in human HepG2(2.2.15) cells assessed as reduction of infectious virus titer treated daily for 9 days by quantitative Southern blot hybri, EC90=0.9μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against lamivudine-resistant Hepatitis B virus harboring reverse transcriptase L180M mutant infected in human HepG2(2.2.15) cells assessed as inhibition of virus replication treated daily for 9 days by quantitative blot hybridization me, EC50=1.5μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against lamivudine-resistant Hepatitis B virus harboring reverse transcriptase L180M mutant infected in human HepG2(2.2.15) cells assessed as reduction of infectious virus titer treated daily for 9 days by quantitative Southern blot hyb, EC90=22μM | 21930377 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 BaL infected in HeLa P4/R5 cells assessed as reduction of viral infection incubated for 2 hrs followed by incubated in drug-free medium for 46 hrs by single round infection beta-galactosidase reporter gene assay, EC50=11.3μM | 22352809 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 3B infected in HeLa P4/R5 cells assessed as reduction of viral infection incubated for 2 hrs followed by incubated in drug-free medium for 46 hrs by single round infection beta-galactosidase reporter gene assay, EC50=32.7μM | 22352809 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 BaL assessed as inhibition of infection in HeLa P4/R5 cells expressing CD4 receptor and beta-gal incubated for 2 hrs followed by compound-wash out measured after 46 hrs by single round infection MAGI assay, EC50=11.4μM | 22533850 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 3B assessed as inhibition of infection in HeLa P4/R5 cells expressing CD4 receptor and beta-gal incubated for 2 hrs followed by compound-wash out measured after 46 hrs by single round infection MAGI assay, EC50=32.7μM | 22533850 | ||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against HIV-1 subtype 3B infected in CEM-SS cells assessed as inhibition of viral replication after 6 days by XTT assay, EC50=0.2μM | 22858097 | ||
| HuH7 | Antiviral assay | Antiviral activity against wild type Hepatitis B virus infected in human HuH7 cells, EC50=0.056μM | 23237841 | |||
| HuH7 | Antiviral assay | Antiviral activity against wild type Hepatitis B virus infected in human HuH7 cells, EC90=0.142μM | 23237841 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as protection against viral-induced cytotoxicity after 5 days by MTT assay, EC50=1.8μM | 24119448 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as protection against viral-induced cytotoxicity after 5 days by MTT assay, EC50=5.9μM | 24119448 | ||
| CEM | Antiviral assay | 4 days | Antiviral activity against HIV1 3B infected in human CEM cells assessed as virus-induced giant cell formation after 4 days by microscopic analysis, EC50=0.099μM | 24177359 | ||
| CEM | Antiviral assay | 4 days | Antiviral activity against HIV2 ROD infected in human CEM cells assessed as virus-induced giant cell formation after 4 days by microscopic analysis, EC50=0.18μM | 24177359 | ||
| HOG.R5 | Antiviral assay | Antiviral activity against HIV-1 3B/H9 infected in human HOG.R5 cells transfected with HIV1 LTR-GFP assessed as inhibition of viral replication by fluorescence analysis, IC50=1.2μM | 24404757 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathicity measured 5 days post viral infection by MTT assay, EC50=2.442μM | 24794751 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as inhibition of virus-induced cytopathicity measured 5 days post viral infection by MTT assay, EC50=7.2μM | 24794751 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against Human immunodeficiency virus 1 3b infected in human MT4 cells assessed as inhibition of virus replication after 5 days by MTT assay, IC50=3.9μM | 24926807 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against Human immunodeficiency virus type 2 ROD infected in human MT4 cells assessed as inhibition of virus replication after 5 days by MTT assay, IC50=15.5μM | 24926807 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathogenicity after 5 days by MTT assay, IC50=3.89μM | 24952305 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as inhibition of virus-induced cytopathogenicity after 5 days by MTT assay, IC50=15.53μM | 24952305 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against hepatitis B virus infected in human HepG2.2.15 cells assessed as inhibition of viral replication, IC50=14.17μM | 25127104 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced syncytium formation after 5 days by MTT assay, EC50=2.22μM | 25240095 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as inhibition of virus-induced syncytium formation after 5 days by MTT assay, EC50=8.81μM | 25240095 | ||
| HepG2(2.2.15) | Function assay | 0.75 to 3 uM | 72 hrs | Decrease in HBV viral DNA replication in human HepG2(2.2.15) cells at 0.75 to 3 uM after 72 hrs by real-time PCR analysis, IC50=7.63μM | 25461891 | |
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV-1 3B infected in human MT4 cells assessed as reduction of virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.24μM | 25537532 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV-2 ROD infected in human MT4 cells assessed as reduction of virus-induced cytopathic effect after 5 days by MTT assay, EC50=8.79μM | 25537532 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.239μM | 25626145 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected infected in human MT4 cells assessed as protection against virus-induced cytotoxicity after 5 days by MTT assay, EC50=0.89μM | 25682562 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as protection against virus-induced cytotoxicity after 5 days by MTT assay, EC50=3.56μM | 25682562 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.2μM | 25707013 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2.2.15 cells assessed as inhibition of HBV DNA replication by real-time fluorescence quantitative PCR analysis, IC50=6.86μM | 25847765 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1-3B infected in human MT4 cells assessed as inhibition of virus induced cell death measured after 5 days of infection by MTT assay, EC50=3.9μM | 25946116 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2-ROD infected in human MT4 cells assessed as inhibition of virus induced cell death measured after 5 days of infection by MTT assay, EC50=15.5μM | 25946116 | ||
| HepG22.2.15 | Antiviral assay | 3 days | Antiviral activity against HBV infected in human HepG22.2.15 cells after 3 days by neutral red dye-based plaque reduction assay, IC50=0.039μM | 26260336 | ||
| U87 | Antiviral assay | 10 uM | 4 days | Antiviral activity against HIV-1 NL4-3 infected in human U87 cells expressing CD4.CXCR4 assessed as inhibition of p24 production at 10 uM measured after 4 days by p24 ELISA | 27091070 | |
| U87 | Antiviral assay | 100 uM | 4 days | Antiviral activity against HIV-1 NL4-3 infected in human U87 cells expressing CD4.CXCR4 assessed as inhibition of p24 production at 100 uM measured after 4 days by p24 ELISA | 27091070 | |
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B expressing wild type reverse transcriptase infected in human MT4 cells assessed as protection against virus-induced cytopathic effect after 5 days by MTT assay, EC50=3.1μM | 27267005 | ||
| HepG2.2.15.7 | Antiviral assay | 9 days | Antiviral activity against HBV infected in human HepG2.2.15.7 cells assessed as inhibition of viral DNA level after 9 days by real time PCR method, EC50=0.03μM | 27426303 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B harboring reverse transcriptase infected in human MT4 cells assessed as protection against virus induced cytotoxicity after 5 days by MTT assay, EC50=2.24μM | 27541578 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD harboring reverse transcriptase infected in human MT4 cells assessed as protection against virus induced cytotoxicity after 5 days by MTT assay, EC50=8.79μM | 27541578 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring intracellular viral DNA copy number after 6 days by real-time qPCR method, EC50<0.01μM | 27748590 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring extracellular viral DNA copy number after 6 days by real-time qPCR method, EC50=0.01μM | 27748590 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as protection against virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.22μM | 27748590 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as protection against virus-induced cytopathic effect after 5 days by MTT assay, EC50=8.81μM | 27748590 | ||
| Huh7 | Antiviral assay | Antiviral activity against lamivudine-resistant wild type hepatitis B virus infected in human Huh7 cells assessed as reduction in secreted DNA levels measured on day 7 by real-time PCR analysis, EC50=0.055μM | 28082068 | |||
| HepG2.215 | Antiviral assay | Antiviral activity against hepatitis B virus infected in human HepG2.215 cells assessed as reduction in viral DNA replication measured on day 7 by real time PCR analysis, EC50=0.1μM | 28082068 | |||
| Huh7 | Antiviral assay | Antiviral activity against lamivudine-resistant wild type hepatitis B virus infected in human Huh7 cells assessed as reduction in intracellular DNA levels measured on day 7 by real-time PCR analysis, EC50=0.237μM | 28082068 | |||
| HepG2.215 | Antiviral assay | Antiviral activity against hepatitis B virus infected in human HepG2.215 cells assessed as reduction in viral DNA replication measured on day 7 by real time PCR analysis, EC90=2μM | 28082068 | |||
| HepAD38 | Antiviral assay | 5 days | Antiviral activity against HBV infected in human HepAD38 cells assessed as inhibition of DNA replication after 5 days by RT-PCR method, EC50=0.04μM | 28094179 | ||
| P4R5 MAGI | Function assay | 2 hrs | Inhibition of HIV1 BaL dinucleoside reverse transcriptase infected in human P4R5 MAGI cells assessed as reduction in viral infection after 2 hrs by Galacto-Star beta-galactosidase reporter gene assay, EC50=11.3μM | 28351588 | ||
| P4R5 MAGI | Function assay | 2 hrs | Inhibition of HIV1 3B dinucleoside reverse transcriptase infected in human P4R5 MAGI cells assessed as reduction in viral infection after 2 hrs by Galacto-Star beta-galactosidase reporter gene assay, EC50=32.7μM | 28351588 | ||
| HepG2.2.15 | Antiviral assay | 8 days | Antiviral activity against Hepatitis B virus infected in human HepG2.2.15 cells assessed as inhibition of viral DNA replication after 8 days by real time fluorescent PCR method, IC50=0.5μM | 28494252 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.54μM | 28659246 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring intracellular viral DNA copy number after 6 days byr by real-time qPCR assay, EC50=0.00887μM | 28682067 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring extracellular viral DNA copy number after 6 days by real-time qPCR method, EC50=0.02μM | 28682067 | ||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in intracellular viral DNA level by RT-PCR method, EC50<0.01μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in viral replication by RT-PCR method, EC50=0.04μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in extracellular viral DNA level by RT-PCR method, EC50=0.04μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in viral replication by RT-PCR method, EC90=0.3μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in extracellular viral DNA level by RT-PCR method, EC90=0.3μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in intracellular viral DNA level by RT-PCR method, EC90=0.7μM | 28688280 | |||
| SupT1 | Antiviral assay | 48 hrs | Antiviral activity against VSV-G pseudotyped HIV1 infected in human SupT1 cells after 48 hrs by luciferase reporter gene assay, IC50=0.1μM | 29016131 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV infected in human HepG2(2.2.15) cells assessed as inhibition of viral DNA replication after 6 days by RT-PCR method, IC50<0.1μM | 29288943 | ||
| A549 | Antiviral assay | Antiviral activity against HIV1 virions derived from HIV/VSG-G or HIV/HA virions infected 293T cells using human A549 cells as target cells assessed as inhibition of viral replication using compound pre-mixed virions followed by incubation with target cel, IC50=0.51μM | 29699924 | |||
| HepG2.2.15 | Antiviral assay | 6 days | Antiviral activity against Hepatitis B virus infected in human HepG2.2.15 cells assessed as reduction in extracellular viral DNA levels after 6 days by qPCR/TaqMan assay, IC50=0.025μM | 30286292 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 RES056 containing reverse transcriptase K103N + Y181C mutant infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 5 days by MTT assay, EC50=7.77μM | 30606670 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 5 days by MTT assay, EC50=12.8μM | 30606670 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytotoxicity after 5 days by MTT assay, EC50=6.41μM | 30721060 | ||
| HepG2.2.15 | Function assay | 4 uM | 6 days | Inhibition of capsid protein in Hepatitis B virus infected in human HepG2.2.15 cells assessed as reduction in viral DNA level at 4 uM supplemented with fresh medium containing compound every 2 days and measured after 6 days by RT-PCR analysis, IC50<0.1μM | 31091479 | |
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as reduction in cytoplasmic DNA synthesis by reed and munch method, IC50=2.22μM | 31223460 | |||
| C8166 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 3B infected in human C8166 cells assessed as inhibition of syncytial cell formation incubated for 72 hrs, EC50=0.3μM | 31310115 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase F227L/V106A double mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=1.92μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase L100I mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.14μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase E138K mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.58μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase K103N mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.7μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV-1 3B infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.88μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase Y188L mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=4.45μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase Y181C mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=4.54μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 RES056 harboring reverse transcriptase K103N/Y181C double mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=5.54μM | 31434039 | ||
| PBMC | Antiviral assay | Antiviral activity in PBM cells expressed as IC50, IC50=0.002μM | ChEMBL | |||
| HepG2.2.15 | Antiviral assay | Antiviral activity against hepatitis B virus in human hepatoma cell line (2.2.15 cells), EC50=0.01μM | ChEMBL | |||
| HepG2.2.15 | Function assay | Effective concentration necessary to decrease extracellular Hepatitis B virus DNA levels by 50% in human hepatoblastoma cell line (2.2.15 cells) in vitro, EC50=0.038μM | ChEMBL | |||
| PBMC | Function assay | Compound was tested for its anti-HIV activity against HIV-1 LAI in human peripheral blood mononuclear (PBM) cells, EC50=0.06μM | ChEMBL | |||
| HepG2.2.15 | Function assay | Effective concentration necessary to decrease extracellular Hepatitis B virus DNA levels by 90% in human hepatoblastoma cell line (2.2.15 cells) in vitro, EC90=0.16μM | ChEMBL | |||
| HepG2.2.15 | Function assay | Tested for the effective concentration required to inhibit HBV in HepG2.2.15 human liver cells, EC50=0.2μM | ChEMBL | |||
| PBMC | Antiviral assay | Antiviral activity in PBM cells, IC50=0.21μM | ChEMBL | |||
| PBMC | Antiviral assay | Antiviral activity in PBM cells expressed as IC50, IC50=0.21μM | ChEMBL | |||
| MT-4 | Antiviral assay | Antiviral activity in MT-4 cells, IC50=0.37μM | ChEMBL | |||
| MT-4 | Antiviral assay | Antiviral activity in MT-4 cells expressed as IC50, IC50=0.37μM | ChEMBL | |||
| MT-4 | Antiviral assay | Antiviral activity in MT-4 cells expressed as IC50, IC50=0.61μM | ChEMBL | |||
| MT-4 | Function assay | Compound was tested for its anti-HIV activity in acutely infected MT-4 cells, EC50=7μM | ChEMBL | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 45 mg/mL
(196.28 mM)
Water : 45 mg/mL Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 229.26 | Formula | C8H11N3O3S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 134678-17-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GR109714X, BCH-189 | Smiles | C1C(OC(S1)CO)N2C=CC(=NC2=O)N | ||
Mechanism of Action
| Targets/IC50/Ki |
Reverse transcriptase
|
|---|---|
| In vitro |
Lamivudine’s anti- HBV activity, like its anti-HIV activity, has been shown to depend on the ability of LMV-TP to serve as both substrate and inhibitor of the DNA- and RNA-dependent polymerase activities of the HBV P gene product. This compound owes its activity to the remarkably broad substrate specificity of deoxycytidine kinase and the unusual substrate preference of the HBV polymerases for dNTPs with the unnatural L-conformation, whereas the anti-HBV activity of PCV appears to depend on several factors including optimal phosphorylation (sufficient for antiviral activity but not cytotoxicity) by key cellular enzymes, the long intracellular half-life of PCV-TP and the ability of PCV-TP to inhibit the HBV RT priming reaction as well as RT and DNA polymerase activity. This compound and Penciclovir inhibits duck hepatitis B virus (DHBV) replication to a comparable extent when used alone, and in combination, the two nucleoside analogs acts synergistically over a wide range of clinically relevant concentrations. It combined with Penciclovir is more effective in reducing the normally recalcitrant viral covalently closed circular (CCC) DNA form of DHBV than either drug alone. It inhibits p24 antigen production by HIV-I in PBMC, with ED50s ranging from 0.07 μM to 0.2 μM. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06056596 | Recruiting | Retinal Detachment|Rhegmatogenous Retinal Detachment |
University of Wisconsin Madison |
January 30 2024 | Early Phase 1 |
| NCT04133012 | Completed | HIV-1 Infection |
ANRS Emerging Infectious Diseases|ViiV Healthcare |
February 10 2020 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































