|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C8H11N3O3S |
|||
| Molecular Weight | 229.26 | CAS No. | 134678-17-4 | |
| Solubility (25°C)* | In vitro | DMSO | 46 mg/mL (200.64 mM) | |
| Water | 46 mg/mL (200.64 mM) | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Lamivudine is a potent nucleoside analog reverse transcriptase inhibitor, used for treatment of chronic HBV and HIV/AIDS. It works by blocking the HIV reverse transcriptase and hepatitis B virus polymerase. | |
|---|---|---|
| Targets |
|
|
| In vitro | Lamivudine’s anti- HBV activity, like its anti-HIV activity, has been shown to depend on the ability of LMV-TP to serve as both substrate and inhibitor of the DNA- and RNA-dependent polymerase activities of the HBV P gene product. Lamivudine owes its activity to the remarkably broad substrate specificity of deoxycytidine kinase and the unusual substrate preference of the HBV polymerases for dNTPs with the unnatural L-conformation, whereas the anti-HBV activity of PCV appears to depend on several factors including optimal phosphorylation (sufficient for antiviral activity but not cytotoxicity) by key cellular enzymes, the long intracellular half-life of PCV-TP and the ability of PCV-TP to inhibit the HBV RT priming reaction as well as RT and DNA polymerase activity. [1] Lamivudine and Penciclovir inhibits duck hepatitis B virus (DHBV) replication to a comparable extent when used alone, and in combination, the two nucleoside analogs acts synergistically over a wide range of clinically relevant concentrations. Lamivudine combined with Penciclovir is more effective in reducing the normally recalcitrant viral covalently closed circular (CCC) DNA form of DHBV than either drug alone. [2] Lamivudine inhibits p24 antigen production by HIV-I in PBMC, with ED50s ranging from 0.07 μM to 0.2 μM. [3] |
Protocol (from reference)
| Cell Assay: |
|
|---|
References
Customer Product Validation
-
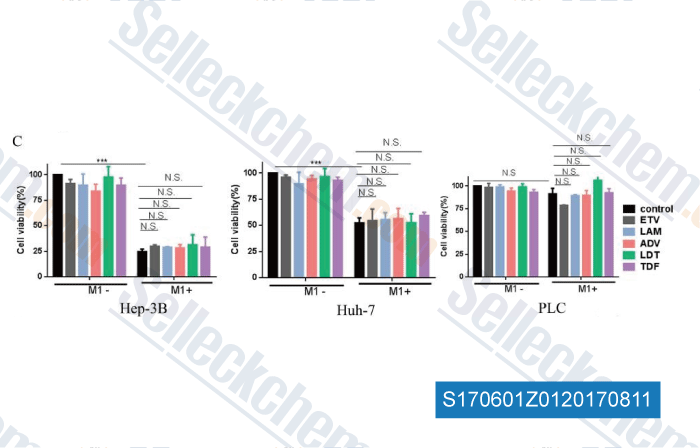
Data from [Data independently produced by , , Oncotarget, 2017, 8(15):24694-24705]
Selleck's Lamivudine has been cited by 16 publications
| Screening of an epigenetic compound library identifies BRD4 as a potential antiviral target for hepatitis B virus covalently closed circular DNA transcription [ Antiviral Res, 2023, 211:105552] | PubMed: 36737008 |
| Farnesoid X receptor alpha ligands inhibit HDV in vitro replication and virion infectivity [ Hepatol Commun, 2023, 7(5)e0078] | PubMed: 37058078 |
| Biological and Structural Analyses of New Potent Allosteric Inhibitors of HIV-1 Integrase [ Antimicrob Agents Chemother, 2023, 67(7):e0046223] | PubMed: 37310224 |
| Kamuvudine-9 Protects Retinal Structure and Function in a Novel Model of Experimental Rhegmatogenous Retinal Detachment [ Invest Ophthalmol Vis Sci, 2023, 64(5):3] | PubMed: 37129905 |
| Reverse Transcriptase Inhibition Disrupts Repeat Element Life Cycle in Colorectal Cancer [ Cancer Discov, 2022, candisc.1117.2021] | PubMed: 35320348 |
| Hepatitis B virus X protein counteracts high mobility group box 1 protein-mediated epigenetic silencing of covalently closed circular DNA [ PLoS Pathog, 2022, 18(6):e1010576] | PubMed: 35679251 |
| Reduction of CD8 T cell functionality but not inhibitory capacity by integrase inhibitors [ J Virol, 2022, JVI0173021] | PubMed: 35019724 |
| Antiretroviral Drugs Regulate Epigenetic Modification of Cardiac Cells Through Modulation of H3K9 and H3K27 Acetylation [ Front Cardiovasc Med, 2021, 8:634774] | PubMed: 33898535 |
| Identification and characterization of a novel hepatitis B virus pregenomic RNA encapsidation inhibitor. [ Antiviral Res, 2020, 175:104709] | PubMed: 31940474 |
| Altered Gut Microbiome under Antiretroviral Therapy: Impact of Efavirenz and Zidovudine [ ACS Infect Dis, 2020, 10.1021/acsinfecdis.0c00536] | PubMed: 33346662 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
