research use only
Gemcitabine DNA Synthesis inhibitor
Cat.No.S1714
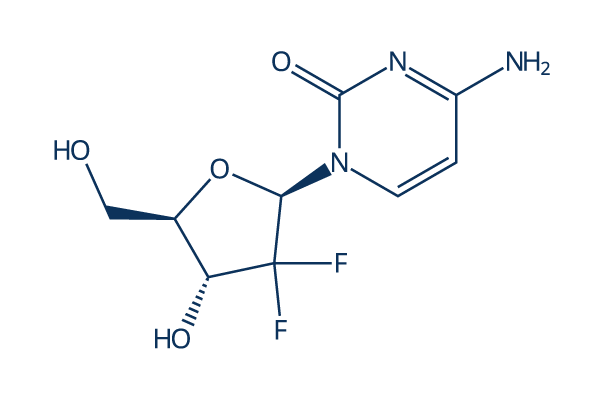
Chemical Structure
Molecular Weight: 263.2
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Triapine (3-AP) Carmofur YK-4-279 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MV-4-11 | Growth inhibition assay | Inhibition of human MV-4-11 cell growth in a cell viability assay, IC50 = 0.0000005 μM. | SANGER | |||
| ES4 | Growth inhibition assay | Inhibition of human ES4 cell growth in a cell viability assay, IC50 = 0.0000007 μM. | SANGER | |||
| ACHN | Growth inhibition assay | Inhibition of human ACHN cell growth in a cell viability assay, IC50 = 0.0000009 μM. | SANGER | |||
| KYSE-510 | Growth inhibition assay | Inhibition of human KYSE-510 cell growth in a cell viability assay, IC50 = 0.000001 μM. | SANGER | |||
| EW-7 | Growth inhibition assay | Inhibition of human EW-7 cell growth in a cell viability assay, IC50 = 0.0000026 μM. | SANGER | |||
| BFTC-905 | Growth inhibition assay | Inhibition of human BFTC-905 cell growth in a cell viability assay, IC50 = 0.0000051 μM. | SANGER | |||
| KE-37 | Growth inhibition assay | Inhibition of human KE-37 cell growth in a cell viability assay, IC50 = 0.0000056 μM. | SANGER | |||
| SBC-5 | Growth inhibition assay | Inhibition of human SBC-5 cell growth in a cell viability assay, IC50 = 0.0000057 μM. | SANGER | |||
| NKM-1 | Growth inhibition assay | Inhibition of human NKM-1 cell growth in a cell viability assay, IC50 = 0.0000071 μM. | SANGER | |||
| RH-1 | Growth inhibition assay | Inhibition of human RH-1 cell growth in a cell viability assay, IC50 = 0.0000072 μM. | SANGER | |||
| ALL-PO | Growth inhibition assay | Inhibition of human ALL-PO cell growth in a cell viability assay, IC50 = 0.0000083 μM. | SANGER | |||
| QIMR-WIL | Growth inhibition assay | Inhibition of human QIMR-WIL cell growth in a cell viability assay, IC50 = 0.0000089 μM. | SANGER | |||
| A375 | Growth inhibition assay | Inhibition of human A375 cell growth in a cell viability assay, IC50 = 0.0000099 μM. | SANGER | |||
| SIG-M5 | Growth inhibition assay | Inhibition of human SIG-M5 cell growth in a cell viability assay, IC50 = 0.0000104 μM. | SANGER | |||
| KGN | Growth inhibition assay | Inhibition of human KGN cell growth in a cell viability assay, IC50 = 0.0000108 μM. | SANGER | |||
| EW-13 | Growth inhibition assay | Inhibition of human EW-13 cell growth in a cell viability assay, IC50 = 0.0000112 μM. | SANGER | |||
| NCI-SNU-1 | Growth inhibition assay | Inhibition of human NCI-SNU-1 cell growth in a cell viability assay, IC50 = 0.000016 μM. | SANGER | |||
| PSN1 | Growth inhibition assay | Inhibition of human PSN1 cell growth in a cell viability assay, IC50 = 0.0000165 μM. | SANGER | |||
| HUTU-80 | Growth inhibition assay | Inhibition of human HUTU-80 cell growth in a cell viability assay, IC50 = 0.0000166 μM. | SANGER | |||
| 786-0 | Growth inhibition assay | Inhibition of human 786-0 cell growth in a cell viability assay, IC50 = 0.000023 μM. | SANGER | |||
| EW-16 | Growth inhibition assay | Inhibition of human EW-16 cell growth in a cell viability assay, IC50 = 0.000023 μM. | SANGER | |||
| ES1 | Growth inhibition assay | Inhibition of human ES1 cell growth in a cell viability assay, IC50 = 0.0000268 μM. | SANGER | |||
| RKO | Growth inhibition assay | Inhibition of human RKO cell growth in a cell viability assay, IC50 = 0.0000278 μM. | SANGER | |||
| ESS-1 | Growth inhibition assay | Inhibition of human ESS-1 cell growth in a cell viability assay, IC50 = 0.0000286 μM. | SANGER | |||
| SK-UT-1 | Growth inhibition assay | Inhibition of human SK-UT-1 cell growth in a cell viability assay, IC50 = 0.0000297 μM. | SANGER | |||
| LB2241-RCC | Growth inhibition assay | Inhibition of human LB2241-RCC cell growth in a cell viability assay, IC50 = 0.0000318 μM. | SANGER | |||
| CHL-1 | Growth inhibition assay | Inhibition of human CHL-1 cell growth in a cell viability assay, IC50 = 0.0000324 μM. | SANGER | |||
| SW1783 | Growth inhibition assay | Inhibition of human SW1783 cell growth in a cell viability assay, IC50 = 0.0000336 μM. | SANGER | |||
| MEL-JUSO | Growth inhibition assay | Inhibition of human MEL-JUSO cell growth in a cell viability assay, IC50 = 0.0000391 μM. | SANGER | |||
| HT-29 | Growth inhibition assay | Inhibition of human HT-29 cell growth in a cell viability assay, IC50 = 0.0000413 μM. | SANGER | |||
| SNG-M | Growth inhibition assay | Inhibition of human SNG-M cell growth in a cell viability assay, IC50 = 0.0000425 μM. | SANGER | |||
| TE-15 | Growth inhibition assay | Inhibition of human TE-15 cell growth in a cell viability assay, IC50 = 0.0000464 μM. | SANGER | |||
| HOS | Growth inhibition assay | Inhibition of human HOS cell growth in a cell viability assay, IC50 = 0.000048 μM. | SANGER | |||
| BB65-RCC | Growth inhibition assay | Inhibition of human BB65-RCC cell growth in a cell viability assay, IC50 = 0.0000512 μM. | SANGER | |||
| HCE-4 | Growth inhibition assay | Inhibition of human HCE-4 cell growth in a cell viability assay, IC50 = 0.0000528 μM. | SANGER | |||
| MHH-ES-1 | Growth inhibition assay | Inhibition of human MHH-ES-1 cell growth in a cell viability assay, IC50 = 0.0000531 μM. | SANGER | |||
| RPMI-7951 | Growth inhibition assay | Inhibition of human RPMI-7951 cell growth in a cell viability assay, IC50 = 0.0000541 μM. | SANGER | |||
| IST-SL2 | Growth inhibition assay | Inhibition of human IST-SL2 cell growth in a cell viability assay, IC50 = 0.0000584 μM. | SANGER | |||
| CMK | Growth inhibition assay | Inhibition of human CMK cell growth in a cell viability assay, IC50 = 0.0000586 μM. | SANGER | |||
| GR-ST | Growth inhibition assay | Inhibition of human GR-ST cell growth in a cell viability assay, IC50 = 0.0000595 μM. | SANGER | |||
| NALM-6 | Growth inhibition assay | Inhibition of human NALM-6 cell growth in a cell viability assay, IC50 = 0.0000622 μM. | SANGER | |||
| RPMI-6666 | Growth inhibition assay | Inhibition of human RPMI-6666 cell growth in a cell viability assay, IC50 = 0.0000652 μM. | SANGER | |||
| LC-2-ad | Growth inhibition assay | Inhibition of human LC-2-ad cell growth in a cell viability assay, IC50 = 0.0000653 μM. | SANGER | |||
| ARH-77 | Growth inhibition assay | Inhibition of human ARH-77 cell growth in a cell viability assay, IC50 = 0.0000711 μM. | SANGER | |||
| IST-MEL1 | Growth inhibition assay | Inhibition of human IST-MEL1 cell growth in a cell viability assay, IC50 = 0.0000726 μM. | SANGER | |||
| SW1710 | Growth inhibition assay | Inhibition of human SW1710 cell growth in a cell viability assay, IC50 = 0.0000751 μM. | SANGER | |||
| DEL | Growth inhibition assay | Inhibition of human DEL cell growth in a cell viability assay, IC50 = 0.0000887 μM. | SANGER | |||
| AGS | Growth inhibition assay | Inhibition of human AGS cell growth in a cell viability assay, IC50 = 0.0000902 μM. | SANGER | |||
| NCI-H2122 | Growth inhibition assay | Inhibition of human NCI-H2122 cell growth in a cell viability assay, IC50 = 0.0000945 μM. | SANGER | |||
| HSC-4 | Growth inhibition assay | Inhibition of human HSC-4 cell growth in a cell viability assay, IC50 = 0.0001022 μM. | SANGER | |||
| AM-38 | Growth inhibition assay | Inhibition of human AM-38 cell growth in a cell viability assay, IC50 = 0.0001215 μM. | SANGER | |||
| 769-P | Growth inhibition assay | Inhibition of human 769-P cell growth in a cell viability assay, IC50 = 0.0001231 μM. | SANGER | |||
| RT-112 | Growth inhibition assay | Inhibition of human RT-112 cell growth in a cell viability assay, IC50 = 0.0001273 μM. | SANGER | |||
| MCF7 | Growth inhibition assay | Inhibition of human MCF7 cell growth in a cell viability assay, IC50 = 0.0001359 μM. | SANGER | |||
| IGROV-1 | Growth inhibition assay | Inhibition of human IGROV-1 cell growth in a cell viability assay, IC50 = 0.000145 μM. | SANGER | |||
| OCI-AML2 | Growth inhibition assay | Inhibition of human OCI-AML2 cell growth in a cell viability assay, IC50 = 0.0001466 μM. | SANGER | |||
| NCI-H1299 | Growth inhibition assay | Inhibition of human NCI-H1299 cell growth in a cell viability assay, IC50 = 0.0001566 μM. | SANGER | |||
| A431 | Growth inhibition assay | Inhibition of human A431 cell growth in a cell viability assay, IC50 = 0.0001831 μM. | SANGER | |||
| SW982 | Growth inhibition assay | Inhibition of human SW982 cell growth in a cell viability assay, IC50 = 0.0002133 μM. | SANGER | |||
| BB30-HNC | Growth inhibition assay | Inhibition of human BB30-HNC cell growth in a cell viability assay, IC50 = 0.0002312 μM. | SANGER | |||
| ACN | Growth inhibition assay | Inhibition of human ACN cell growth in a cell viability assay, IC50 = 0.0002436 μM. | SANGER | |||
| 647-V | Growth inhibition assay | Inhibition of human 647-V cell growth in a cell viability assay, IC50 = 0.0002481 μM. | SANGER | |||
| SK-PN-DW | Growth inhibition assay | Inhibition of human SK-PN-DW cell growth in a cell viability assay, IC50 = 0.0002656 μM. | SANGER | |||
| LCLC-97TM1 | Growth inhibition assay | Inhibition of human LCLC-97TM1 cell growth in a cell viability assay, IC50 = 0.0002673 μM. | SANGER | |||
| LB1047-RCC | Growth inhibition assay | Inhibition of human LB1047-RCC cell growth in a cell viability assay, IC50 = 0.0002688 μM. | SANGER | |||
| A2780 | Growth inhibition assay | Inhibition of human A2780 cell growth in a cell viability assay, IC50 = 0.0002702 μM. | SANGER | |||
| C-33-A | Growth inhibition assay | Inhibition of human C-33-A cell growth in a cell viability assay, IC50 = 0.0002733 μM. | SANGER | |||
| NCI-H2228 | Growth inhibition assay | Inhibition of human NCI-H2228 cell growth in a cell viability assay, IC50 = 0.000314 μM. | SANGER | |||
| TE-5 | Growth inhibition assay | Inhibition of human TE-5 cell growth in a cell viability assay, IC50 = 0.0003157 μM. | SANGER | |||
| HC-1 | Growth inhibition assay | Inhibition of human HC-1 cell growth in a cell viability assay, IC50 = 0.0003273 μM. | SANGER | |||
| SK-MES-1 | Growth inhibition assay | Inhibition of human SK-MES-1 cell growth in a cell viability assay, IC50 = 0.0003279 μM. | SANGER | |||
| NCI-H1355 | Growth inhibition assay | Inhibition of human NCI-H1355 cell growth in a cell viability assay, IC50 = 0.0003806 μM. | SANGER | |||
| YKG-1 | Growth inhibition assay | Inhibition of human YKG-1 cell growth in a cell viability assay, IC50 = 0.0004194 μM. | SANGER | |||
| RS4-11 | Growth inhibition assay | Inhibition of human RS4-11 cell growth in a cell viability assay, IC50 = 0.0004326 μM. | SANGER | |||
| Daoy | Growth inhibition assay | Inhibition of human Daoy cell growth in a cell viability assay, IC50 = 0.0004565 μM. | SANGER | |||
| A3-KAW | Growth inhibition assay | Inhibition of human A3-KAW cell growth in a cell viability assay, IC50 = 0.0005512 μM. | SANGER | |||
| SK-MEL-30 | Growth inhibition assay | Inhibition of human SK-MEL-30 cell growth in a cell viability assay, IC50 = 0.0005545 μM. | SANGER | |||
| U031 | Growth inhibition assay | Inhibition of human U031 cell growth in a cell viability assay, IC50 = 0.0005647 μM. | SANGER | |||
| SK-LMS-1 | Growth inhibition assay | Inhibition of human SK-LMS-1 cell growth in a cell viability assay, IC50 = 0.0005776 μM. | SANGER | |||
| ES6 | Growth inhibition assay | Inhibition of human ES6 cell growth in a cell viability assay, IC50 = 0.0005856 μM. | SANGER | |||
| EoL-1-cell | Growth inhibition assay | Inhibition of human EoL-1-cell cell growth in a cell viability assay, IC50 = 0.0006162 μM. | SANGER | |||
| NCI-H2009 | Growth inhibition assay | Inhibition of human NCI-H2009 cell growth in a cell viability assay, IC50 = 0.0006187 μM. | SANGER | |||
| A4-Fuk | Growth inhibition assay | Inhibition of human A4-Fuk cell growth in a cell viability assay, IC50 = 0.0006263 μM. | SANGER | |||
| KYSE-270 | Growth inhibition assay | Inhibition of human KYSE-270 cell growth in a cell viability assay, IC50 = 0.0006341 μM. | SANGER | |||
| SK-LU-1 | Growth inhibition assay | Inhibition of human SK-LU-1 cell growth in a cell viability assay, IC50 = 0.0006552 μM. | SANGER | |||
| SW872 | Growth inhibition assay | Inhibition of human SW872 cell growth in a cell viability assay, IC50 = 0.0007647 μM. | SANGER | |||
| ES8 | Growth inhibition assay | Inhibition of human ES8 cell growth in a cell viability assay, IC50 = 0.0007802 μM. | SANGER | |||
| G-402 | Growth inhibition assay | Inhibition of human G-402 cell growth in a cell viability assay, IC50 = 0.0007844 μM. | SANGER | |||
| ATN-1 | Growth inhibition assay | Inhibition of human ATN-1 cell growth in a cell viability assay, IC50 = 0.0008069 μM. | SANGER | |||
| DoTc2-4510 | Growth inhibition assay | Inhibition of human DoTc2-4510 cell growth in a cell viability assay, IC50 = 0.0009012 μM. | SANGER | |||
| MES-SA | Growth inhibition assay | Inhibition of human MES-SA cell growth in a cell viability assay, IC50 = 0.0009049 μM. | SANGER | |||
| SF268 | Growth inhibition assay | Inhibition of human SF268 cell growth in a cell viability assay, IC50 = 0.0009274 μM. | SANGER | |||
| SF539 | Growth inhibition assay | Inhibition of human SF539 cell growth in a cell viability assay, IC50 = 0.001023 μM. | SANGER | |||
| NB69 | Growth inhibition assay | Inhibition of human NB69 cell growth in a cell viability assay, IC50 = 0.001046 μM. | SANGER | |||
| 8505C | Growth inhibition assay | Inhibition of human 8505C cell growth in a cell viability assay, IC50 = 0.001063 μM. | SANGER | |||
| CAL-12T | Growth inhibition assay | Inhibition of human CAL-12T cell growth in a cell viability assay, IC50 = 0.001084 μM. | SANGER | |||
| BHY | Growth inhibition assay | Inhibition of human BHY cell growth in a cell viability assay, IC50 = 0.001141 μM. | SANGER | |||
| LB647-SCLC | Growth inhibition assay | Inhibition of human LB647-SCLC cell growth in a cell viability assay, IC50 = 0.00118 μM. | SANGER | |||
| CAL-62 | Growth inhibition assay | Inhibition of human CAL-62 cell growth in a cell viability assay, IC50 = 0.001215 μM. | SANGER | |||
| MEG-01 | Growth inhibition assay | Inhibition of human MEG-01 cell growth in a cell viability assay, IC50 = 0.001266 μM. | SANGER | |||
| MG-63 | Growth inhibition assay | Inhibition of human MG-63 cell growth in a cell viability assay, IC50 = 0.001335 μM. | SANGER | |||
| SW620 | Growth inhibition assay | Inhibition of human SW620 cell growth in a cell viability assay, IC50 = 0.001346 μM. | SANGER | |||
| A388 | Growth inhibition assay | Inhibition of human A388 cell growth in a cell viability assay, IC50 = 0.001365 μM. | SANGER | |||
| BCPAP | Growth inhibition assay | Inhibition of human BCPAP cell growth in a cell viability assay, IC50 = 0.001452 μM. | SANGER | |||
| P30-OHK | Growth inhibition assay | Inhibition of human P30-OHK cell growth in a cell viability assay, IC50 = 0.001459 μM. | SANGER | |||
| Ca9-22 | Growth inhibition assay | Inhibition of human Ca9-22 cell growth in a cell viability assay, IC50 = 0.001538 μM. | SANGER | |||
| VMRC-RCZ | Growth inhibition assay | Inhibition of human VMRC-RCZ cell growth in a cell viability assay, IC50 = 0.001542 μM. | SANGER | |||
| LOXIMVI | Growth inhibition assay | Inhibition of human LOXIMVI cell growth in a cell viability assay, IC50 = 0.001596 μM. | SANGER | |||
| L-540 | Growth inhibition assay | Inhibition of human L-540 cell growth in a cell viability assay, IC50 = 0.001602 μM. | SANGER | |||
| NTERA-S-cl-D1 | Growth inhibition assay | Inhibition of human NTERA-S-cl-D1 cell growth in a cell viability assay, IC50 = 0.001641 μM. | SANGER | |||
| MFH-ino | Growth inhibition assay | Inhibition of human MFH-ino cell growth in a cell viability assay, IC50 = 0.001656 μM. | SANGER | |||
| Calu-6 | Growth inhibition assay | Inhibition of human Calu-6 cell growth in a cell viability assay, IC50 = 0.001735 μM. | SANGER | |||
| HEL | Growth inhibition assay | Inhibition of human HEL cell growth in a cell viability assay, IC50 = 0.001791 μM. | SANGER | |||
| CAL-33 | Growth inhibition assay | Inhibition of human CAL-33 cell growth in a cell viability assay, IC50 = 0.001893 μM. | SANGER | |||
| HSC-3 | Growth inhibition assay | Inhibition of human HSC-3 cell growth in a cell viability assay, IC50 = 0.001905 μM. | SANGER | |||
| KU812 | Growth inhibition assay | Inhibition of human KU812 cell growth in a cell viability assay, IC50 = 0.001913 μM. | SANGER | |||
| EB2 | Growth inhibition assay | Inhibition of human EB2 cell growth in a cell viability assay, IC50 = 0.002012 μM. | SANGER | |||
| SR | Growth inhibition assay | Inhibition of human SR cell growth in a cell viability assay, IC50 = 0.002121 μM. | SANGER | |||
| NCI-H2087 | Growth inhibition assay | Inhibition of human NCI-H2087 cell growth in a cell viability assay, IC50 = 0.002143 μM. | SANGER | |||
| H4 | Growth inhibition assay | Inhibition of human H4 cell growth in a cell viability assay, IC50 = 0.002175 μM. | SANGER | |||
| EW-1 | Growth inhibition assay | Inhibition of human EW-1 cell growth in a cell viability assay, IC50 = 0.002223 μM. | SANGER | |||
| MC-IXC | Growth inhibition assay | Inhibition of human MC-IXC cell growth in a cell viability assay, IC50 = 0.002264 μM. | SANGER | |||
| NCI-H727 | Growth inhibition assay | Inhibition of human NCI-H727 cell growth in a cell viability assay, IC50 = 0.002506 μM. | SANGER | |||
| MRK-nu-1 | Growth inhibition assay | Inhibition of human MRK-nu-1 cell growth in a cell viability assay, IC50 = 0.002567 μM. | SANGER | |||
| COLO-668 | Growth inhibition assay | Inhibition of human COLO-668 cell growth in a cell viability assay, IC50 = 0.00266 μM. | SANGER | |||
| CGTH-W-1 | Growth inhibition assay | Inhibition of human CGTH-W-1 cell growth in a cell viability assay, IC50 = 0.002723 μM. | SANGER | |||
| CHP-212 | Growth inhibition assay | Inhibition of human CHP-212 cell growth in a cell viability assay, IC50 = 0.002752 μM. | SANGER | |||
| GI-1 | Growth inhibition assay | Inhibition of human GI-1 cell growth in a cell viability assay, IC50 = 0.002764 μM. | SANGER | |||
| HCC1806 | Growth inhibition assay | Inhibition of human HCC1806 cell growth in a cell viability assay, IC50 = 0.002908 μM. | SANGER | |||
| HLE | Growth inhibition assay | Inhibition of human HLE cell growth in a cell viability assay, IC50 = 0.003004 μM. | SANGER | |||
| HSC-2 | Growth inhibition assay | Inhibition of human HSC-2 cell growth in a cell viability assay, IC50 = 0.00303 μM. | SANGER | |||
| DMS-273 | Growth inhibition assay | Inhibition of human DMS-273 cell growth in a cell viability assay, IC50 = 0.00307 μM. | SANGER | |||
| DU-4475 | Growth inhibition assay | Inhibition of human DU-4475 cell growth in a cell viability assay, IC50 = 0.003143 μM. | SANGER | |||
| LXF-289 | Growth inhibition assay | Inhibition of human LXF-289 cell growth in a cell viability assay, IC50 = 0.003314 μM. | SANGER | |||
| PANC-03-27 | Growth inhibition assay | Inhibition of human PANC-03-27 cell growth in a cell viability assay, IC50 = 0.003513 μM. | SANGER | |||
| GAMG | Growth inhibition assay | Inhibition of human GAMG cell growth in a cell viability assay, IC50 = 0.003739 μM. | SANGER | |||
| NCI-H522 | Growth inhibition assay | Inhibition of human NCI-H522 cell growth in a cell viability assay, IC50 = 0.004337 μM. | SANGER | |||
| SW626 | Growth inhibition assay | Inhibition of human SW626 cell growth in a cell viability assay, IC50 = 0.004464 μM. | SANGER | |||
| HT-144 | Growth inhibition assay | Inhibition of human HT-144 cell growth in a cell viability assay, IC50 = 0.00492 μM. | SANGER | |||
| MEL-HO | Growth inhibition assay | Inhibition of human MEL-HO cell growth in a cell viability assay, IC50 = 0.005162 μM. | SANGER | |||
| BE-13 | Growth inhibition assay | Inhibition of human BE-13 cell growth in a cell viability assay, IC50 = 0.00521 μM. | SANGER | |||
| VA-ES-BJ | Growth inhibition assay | Inhibition of human VA-ES-BJ cell growth in a cell viability assay, IC50 = 0.005256 μM. | SANGER | |||
| NCI-H441 | Growth inhibition assay | Inhibition of human NCI-H441 cell growth in a cell viability assay, IC50 = 0.005597 μM. | SANGER | |||
| KP-4 | Growth inhibition assay | Inhibition of human KP-4 cell growth in a cell viability assay, IC50 = 0.005611 μM. | SANGER | |||
| LoVo | Growth inhibition assay | Inhibition of human LoVo cell growth in a cell viability assay, IC50 = 0.005714 μM. | SANGER | |||
| HT-1080 | Growth inhibition assay | Inhibition of human HT-1080 cell growth in a cell viability assay, IC50 = 0.005834 μM. | SANGER | |||
| GB-1 | Growth inhibition assay | Inhibition of human GB-1 cell growth in a cell viability assay, IC50 = 0.005845 μM. | SANGER | |||
| IA-LM | Growth inhibition assay | Inhibition of human IA-LM cell growth in a cell viability assay, IC50 = 0.005906 μM. | SANGER | |||
| 8-MG-BA | Growth inhibition assay | Inhibition of human 8-MG-BA cell growth in a cell viability assay, IC50 = 0.00593 μM. | SANGER | |||
| SK-HEP-1 | Growth inhibition assay | Inhibition of human SK-HEP-1 cell growth in a cell viability assay, IC50 = 0.006136 μM. | SANGER | |||
| 697 | Growth inhibition assay | Inhibition of human 697 cell growth in a cell viability assay, IC50 = 0.006247 μM. | SANGER | |||
| KYSE-450 | Growth inhibition assay | Inhibition of human KYSE-450 cell growth in a cell viability assay, IC50 = 0.006315 μM. | SANGER | |||
| HCC2998 | Growth inhibition assay | Inhibition of human HCC2998 cell growth in a cell viability assay, IC50 = 0.006339 μM. | SANGER | |||
| HD-MY-Z | Growth inhibition assay | Inhibition of human HD-MY-Z cell growth in a cell viability assay, IC50 = 0.006679 μM. | SANGER | |||
| OS-RC-2 | Growth inhibition assay | Inhibition of human OS-RC-2 cell growth in a cell viability assay, IC50 = 0.006681 μM. | SANGER | |||
| SF126 | Growth inhibition assay | Inhibition of human SF126 cell growth in a cell viability assay, IC50 = 0.007054 μM. | SANGER | |||
| Ca-Ski | Growth inhibition assay | Inhibition of human Ca-Ski cell growth in a cell viability assay, IC50 = 0.007093 μM. | SANGER | |||
| NCI-H358 | Growth inhibition assay | Inhibition of human NCI-H358 cell growth in a cell viability assay, IC50 = 0.00716 μM. | SANGER | |||
| J82 | Growth inhibition assay | Inhibition of human J82 cell growth in a cell viability assay, IC50 = 0.00741 μM. | SANGER | |||
| NCI-H2342 | Growth inhibition assay | Inhibition of human NCI-H2342 cell growth in a cell viability assay, IC50 = 0.007634 μM. | SANGER | |||
| OVCAR-8 | Growth inhibition assay | Inhibition of human OVCAR-8 cell growth in a cell viability assay, IC50 = 0.007904 μM. | SANGER | |||
| TE-8 | Growth inhibition assay | Inhibition of human TE-8 cell growth in a cell viability assay, IC50 = 0.008001 μM. | SANGER | |||
| ETK-1 | Growth inhibition assay | Inhibition of human ETK-1 cell growth in a cell viability assay, IC50 = 0.008076 μM. | SANGER | |||
| HAL-01 | Growth inhibition assay | Inhibition of human HAL-01 cell growth in a cell viability assay, IC50 = 0.008195 μM. | SANGER | |||
| KYSE-150 | Growth inhibition assay | Inhibition of human KYSE-150 cell growth in a cell viability assay, IC50 = 0.008469 μM. | SANGER | |||
| NCI-H810 | Growth inhibition assay | Inhibition of human NCI-H810 cell growth in a cell viability assay, IC50 = 0.008558 μM. | SANGER | |||
| ONS-76 | Growth inhibition assay | Inhibition of human ONS-76 cell growth in a cell viability assay, IC50 = 0.008677 μM. | SANGER | |||
| NMC-G1 | Growth inhibition assay | Inhibition of human NMC-G1 cell growth in a cell viability assay, IC50 = 0.008762 μM. | SANGER | |||
| C3A | Growth inhibition assay | Inhibition of human C3A cell growth in a cell viability assay, IC50 = 0.008839 μM. | SANGER | |||
| PA-1 | Growth inhibition assay | Inhibition of human PA-1 cell growth in a cell viability assay, IC50 = 0.008993 μM. | SANGER | |||
| SH-4 | Growth inhibition assay | Inhibition of human SH-4 cell growth in a cell viability assay, IC50 = 0.009022 μM. | SANGER | |||
| EFO-27 | Growth inhibition assay | Inhibition of human EFO-27 cell growth in a cell viability assay, IC50 = 0.009046 μM. | SANGER | |||
| CAPAN-1 | Growth inhibition assay | Inhibition of human CAPAN-1 cell growth in a cell viability assay, IC50 = 0.009227 μM. | SANGER | |||
| DU-145 | Growth inhibition assay | Inhibition of human DU-145 cell growth in a cell viability assay, IC50 = 0.00929 μM. | SANGER | |||
| A101D | Growth inhibition assay | Inhibition of human A101D cell growth in a cell viability assay, IC50 = 0.009373 μM. | SANGER | |||
| ST486 | Growth inhibition assay | Inhibition of human ST486 cell growth in a cell viability assay, IC50 = 0.009406 μM. | SANGER | |||
| NCI-H1437 | Growth inhibition assay | Inhibition of human NCI-H1437 cell growth in a cell viability assay, IC50 = 0.009418 μM. | SANGER | |||
| HGC-27 | Growth inhibition assay | Inhibition of human HGC-27 cell growth in a cell viability assay, IC50 = 0.009601 μM. | SANGER | |||
| 8305C | Growth inhibition assay | Inhibition of human 8305C cell growth in a cell viability assay, IC50 = 0.00964 μM. | SANGER | |||
| OCUB-M | Growth inhibition assay | Inhibition of human OCUB-M cell growth in a cell viability assay, IC50 = 0.01003 μM. | SANGER | |||
| COLO-679 | Growth inhibition assay | Inhibition of human COLO-679 cell growth in a cell viability assay, IC50 = 0.01007 μM. | SANGER | |||
| Detroit562 | Growth inhibition assay | Inhibition of human Detroit562 cell growth in a cell viability assay, IC50 = 0.01042 μM. | SANGER | |||
| A204 | Growth inhibition assay | Inhibition of human A204 cell growth in a cell viability assay, IC50 = 0.01116 μM. | SANGER | |||
| NCI-H1734 | Growth inhibition assay | Inhibition of human NCI-H1734 cell growth in a cell viability assay, IC50 = 0.01129 μM. | SANGER | |||
| MC-CAR | Growth inhibition assay | Inhibition of human MC-CAR cell growth in a cell viability assay, IC50 = 0.01158 μM. | SANGER | |||
| NCI-H2170 | Growth inhibition assay | Inhibition of human NCI-H2170 cell growth in a cell viability assay, IC50 = 0.01197 μM. | SANGER | |||
| NCI-SNU-5 | Growth inhibition assay | Inhibition of human NCI-SNU-5 cell growth in a cell viability assay, IC50 = 0.01213 μM. | SANGER | |||
| HCE-T | Growth inhibition assay | Inhibition of human HCE-T cell growth in a cell viability assay, IC50 = 0.01242 μM. | SANGER | |||
| KYSE-180 | Growth inhibition assay | Inhibition of human KYSE-180 cell growth in a cell viability assay, IC50 = 0.01281 μM. | SANGER | |||
| C8166 | Growth inhibition assay | Inhibition of human C8166 cell growth in a cell viability assay, IC50 = 0.01308 μM. | SANGER | |||
| NCI-H460 | Growth inhibition assay | Inhibition of human NCI-H460 cell growth in a cell viability assay, IC50 = 0.01354 μM. | SANGER | |||
| SNU-449 | Growth inhibition assay | Inhibition of human SNU-449 cell growth in a cell viability assay, IC50 = 0.01377 μM. | SANGER | |||
| MDA-MB-468 | Growth inhibition assay | Inhibition of human MDA-MB-468 cell growth in a cell viability assay, IC50 = 0.01412 μM. | SANGER | |||
| COR-L23 | Growth inhibition assay | Inhibition of human COR-L23 cell growth in a cell viability assay, IC50 = 0.01413 μM. | SANGER | |||
| CTV-1 | Growth inhibition assay | Inhibition of human CTV-1 cell growth in a cell viability assay, IC50 = 0.01414 μM. | SANGER | |||
| BL-41 | Growth inhibition assay | Inhibition of human BL-41 cell growth in a cell viability assay, IC50 = 0.01437 μM. | SANGER | |||
| IGR-1 | Growth inhibition assay | Inhibition of human IGR-1 cell growth in a cell viability assay, IC50 = 0.01442 μM. | SANGER | |||
| TK10 | Growth inhibition assay | Inhibition of human TK10 cell growth in a cell viability assay, IC50 = 0.01449 μM. | SANGER | |||
| REH | Growth inhibition assay | Inhibition of human REH cell growth in a cell viability assay, IC50 = 0.01451 μM. | SANGER | |||
| LU-139 | Growth inhibition assay | Inhibition of human LU-139 cell growth in a cell viability assay, IC50 = 0.01459 μM. | SANGER | |||
| KP-N-YS | Growth inhibition assay | Inhibition of human KP-N-YS cell growth in a cell viability assay, IC50 = 0.01497 μM. | SANGER | |||
| PANC-10-05 | Growth inhibition assay | Inhibition of human PANC-10-05 cell growth in a cell viability assay, IC50 = 0.01538 μM. | SANGER | |||
| HL-60 | Growth inhibition assay | Inhibition of human HL-60 cell growth in a cell viability assay, IC50 = 0.01569 μM. | SANGER | |||
| T84 | Growth inhibition assay | Inhibition of human T84 cell growth in a cell viability assay, IC50 = 0.01596 μM. | SANGER | |||
| RPMI-8226 | Growth inhibition assay | Inhibition of human RPMI-8226 cell growth in a cell viability assay, IC50 = 0.01602 μM. | SANGER | |||
| UM-UC-3 | Growth inhibition assay | Inhibition of human UM-UC-3 cell growth in a cell viability assay, IC50 = 0.01616 μM. | SANGER | |||
| TE-10 | Growth inhibition assay | Inhibition of human TE-10 cell growth in a cell viability assay, IC50 = 0.01621 μM. | SANGER | |||
| CAL-148 | Growth inhibition assay | Inhibition of human CAL-148 cell growth in a cell viability assay, IC50 = 0.01723 μM. | SANGER | |||
| BV-173 | Growth inhibition assay | Inhibition of human BV-173 cell growth in a cell viability assay, IC50 = 0.01727 μM. | SANGER | |||
| Calu-3 | Growth inhibition assay | Inhibition of human Calu-3 cell growth in a cell viability assay, IC50 = 0.01729 μM. | SANGER | |||
| RPMI-2650 | Growth inhibition assay | Inhibition of human RPMI-2650 cell growth in a cell viability assay, IC50 = 0.01759 μM. | SANGER | |||
| MKN45 | Growth inhibition assay | Inhibition of human MKN45 cell growth in a cell viability assay, IC50 = 0.01773 μM. | SANGER | |||
| NUGC-3 | Growth inhibition assay | Inhibition of human NUGC-3 cell growth in a cell viability assay, IC50 = 0.01834 μM. | SANGER | |||
| NCI-H520 | Growth inhibition assay | Inhibition of human NCI-H520 cell growth in a cell viability assay, IC50 = 0.01877 μM. | SANGER | |||
| CCRF-CEM | Growth inhibition assay | Inhibition of human CCRF-CEM cell growth in a cell viability assay, IC50 = 0.01885 μM. | SANGER | |||
| NCI-H2405 | Growth inhibition assay | Inhibition of human NCI-H2405 cell growth in a cell viability assay, IC50 = 0.0191 μM. | SANGER | |||
| ES7 | Growth inhibition assay | Inhibition of human ES7 cell growth in a cell viability assay, IC50 = 0.01976 μM. | SANGER | |||
| BPH-1 | Growth inhibition assay | Inhibition of human BPH-1 cell growth in a cell viability assay, IC50 = 0.02028 μM. | SANGER | |||
| SAS | Growth inhibition assay | Inhibition of human SAS cell growth in a cell viability assay, IC50 = 0.0205 μM. | SANGER | |||
| HuCCT1 | Growth inhibition assay | Inhibition of human HuCCT1 cell growth in a cell viability assay, IC50 = 0.02058 μM. | SANGER | |||
| LOUCY | Growth inhibition assay | Inhibition of human LOUCY cell growth in a cell viability assay, IC50 = 0.02066 μM. | SANGER | |||
| NCI-H292 | Growth inhibition assay | Inhibition of human NCI-H292 cell growth in a cell viability assay, IC50 = 0.02079 μM. | SANGER | |||
| G-361 | Growth inhibition assay | Inhibition of human G-361 cell growth in a cell viability assay, IC50 = 0.02107 μM. | SANGER | |||
| M059J | Growth inhibition assay | Inhibition of human M059J cell growth in a cell viability assay, IC50 = 0.02108 μM. | SANGER | |||
| NCI-H1651 | Growth inhibition assay | Inhibition of human NCI-H1651 cell growth in a cell viability assay, IC50 = 0.02111 μM. | SANGER | |||
| KALS-1 | Growth inhibition assay | Inhibition of human KALS-1 cell growth in a cell viability assay, IC50 = 0.02139 μM. | SANGER | |||
| DJM-1 | Growth inhibition assay | Inhibition of human DJM-1 cell growth in a cell viability assay, IC50 = 0.02159 μM. | SANGER | |||
| AU565 | Growth inhibition assay | Inhibition of human AU565 cell growth in a cell viability assay, IC50 = 0.02183 μM. | SANGER | |||
| HCC38 | Growth inhibition assay | Inhibition of human HCC38 cell growth in a cell viability assay, IC50 = 0.02195 μM. | SANGER | |||
| U251 | Growth inhibition assay | Inhibition of human U251 cell growth in a cell viability assay, IC50 = 0.02227 μM. | SANGER | |||
| ABC-1 | Growth inhibition assay | Inhibition of human ABC-1 cell growth in a cell viability assay, IC50 = 0.02265 μM. | SANGER | |||
| SK-NEP-1 | Growth inhibition assay | Inhibition of human SK-NEP-1 cell growth in a cell viability assay, IC50 = 0.02293 μM. | SANGER | |||
| CESS | Growth inhibition assay | Inhibition of human CESS cell growth in a cell viability assay, IC50 = 0.02319 μM. | SANGER | |||
| MIA-PaCa-2 | Growth inhibition assay | Inhibition of human MIA-PaCa-2 cell growth in a cell viability assay, IC50 = 0.02336 μM. | SANGER | |||
| SUP-T1 | Growth inhibition assay | Inhibition of human SUP-T1 cell growth in a cell viability assay, IC50 = 0.02347 μM. | SANGER | |||
| L-428 | Growth inhibition assay | Inhibition of human L-428 cell growth in a cell viability assay, IC50 = 0.02362 μM. | SANGER | |||
| SW954 | Growth inhibition assay | Inhibition of human SW954 cell growth in a cell viability assay, IC50 = 0.02368 μM. | SANGER | |||
| HO-1-N-1 | Growth inhibition assay | Inhibition of human HO-1-N-1 cell growth in a cell viability assay, IC50 = 0.02377 μM. | SANGER | |||
| CHP-126 | Growth inhibition assay | Inhibition of human CHP-126 cell growth in a cell viability assay, IC50 = 0.02414 μM. | SANGER | |||
| HMV-II | Growth inhibition assay | Inhibition of human HMV-II cell growth in a cell viability assay, IC50 = 0.02434 μM. | SANGER | |||
| NB10 | Growth inhibition assay | Inhibition of human NB10 cell growth in a cell viability assay, IC50 = 0.02437 μM. | SANGER | |||
| A172 | Growth inhibition assay | Inhibition of human A172 cell growth in a cell viability assay, IC50 = 0.02471 μM. | SANGER | |||
| MONO-MAC-6 | Growth inhibition assay | Inhibition of human MONO-MAC-6 cell growth in a cell viability assay, IC50 = 0.02484 μM. | SANGER | |||
| NCI-H1650 | Growth inhibition assay | Inhibition of human NCI-H1650 cell growth in a cell viability assay, IC50 = 0.0254 μM. | SANGER | |||
| NH-12 | Growth inhibition assay | Inhibition of human NH-12 cell growth in a cell viability assay, IC50 = 0.0255 μM. | SANGER | |||
| ML-2 | Growth inhibition assay | Inhibition of human ML-2 cell growth in a cell viability assay, IC50 = 0.02574 μM. | SANGER | |||
| MZ2-MEL | Growth inhibition assay | Inhibition of human MZ2-MEL cell growth in a cell viability assay, IC50 = 0.02622 μM. | SANGER | |||
| COLO-684 | Growth inhibition assay | Inhibition of human COLO-684 cell growth in a cell viability assay, IC50 = 0.02641 μM. | SANGER | |||
| HuP-T4 | Growth inhibition assay | Inhibition of human HuP-T4 cell growth in a cell viability assay, IC50 = 0.0273 μM. | SANGER | |||
| SW837 | Growth inhibition assay | Inhibition of human SW837 cell growth in a cell viability assay, IC50 = 0.02762 μM. | SANGER | |||
| MDA-MB-231 | Growth inhibition assay | Inhibition of human MDA-MB-231 cell growth in a cell viability assay, IC50 = 0.02778 μM. | SANGER | |||
| KYSE-140 | Growth inhibition assay | Inhibition of human KYSE-140 cell growth in a cell viability assay, IC50 = 0.02791 μM. | SANGER | |||
| NOMO-1 | Growth inhibition assay | Inhibition of human NOMO-1 cell growth in a cell viability assay, IC50 = 0.02868 μM. | SANGER | |||
| GP5d | Growth inhibition assay | Inhibition of human GP5d cell growth in a cell viability assay, IC50 = 0.02872 μM. | SANGER | |||
| COR-L105 | Growth inhibition assay | Inhibition of human COR-L105 cell growth in a cell viability assay, IC50 = 0.02942 μM. | SANGER | |||
| LS-411N | Growth inhibition assay | Inhibition of human LS-411N cell growth in a cell viability assay, IC50 = 0.02988 μM. | SANGER | |||
| NY | Growth inhibition assay | Inhibition of human NY cell growth in a cell viability assay, IC50 = 0.03018 μM. | SANGER | |||
| NCI-H2030 | Growth inhibition assay | Inhibition of human NCI-H2030 cell growth in a cell viability assay, IC50 = 0.03045 μM. | SANGER | |||
| CCF-STTG1 | Growth inhibition assay | Inhibition of human CCF-STTG1 cell growth in a cell viability assay, IC50 = 0.03142 μM. | SANGER | |||
| NCI-H1703 | Growth inhibition assay | Inhibition of human NCI-H1703 cell growth in a cell viability assay, IC50 = 0.03178 μM. | SANGER | |||
| TUR | Growth inhibition assay | Inhibition of human TUR cell growth in a cell viability assay, IC50 = 0.03203 μM. | SANGER | |||
| NOS-1 | Growth inhibition assay | Inhibition of human NOS-1 cell growth in a cell viability assay, IC50 = 0.03244 μM. | SANGER | |||
| A2058 | Growth inhibition assay | Inhibition of human A2058 cell growth in a cell viability assay, IC50 = 0.03283 μM. | SANGER | |||
| LCLC-103H | Growth inhibition assay | Inhibition of human LCLC-103H cell growth in a cell viability assay, IC50 = 0.03325 μM. | SANGER | |||
| NCI-H510A | Growth inhibition assay | Inhibition of human NCI-H510A cell growth in a cell viability assay, IC50 = 0.03327 μM. | SANGER | |||
| BC-1 | Growth inhibition assay | Inhibition of human BC-1 cell growth in a cell viability assay, IC50 = 0.03377 μM. | SANGER | |||
| SK-CO-1 | Growth inhibition assay | Inhibition of human SK-CO-1 cell growth in a cell viability assay, IC50 = 0.03401 μM. | SANGER | |||
| A673 | Growth inhibition assay | Inhibition of human A673 cell growth in a cell viability assay, IC50 = 0.03417 μM. | SANGER | |||
| VM-CUB-1 | Growth inhibition assay | Inhibition of human VM-CUB-1 cell growth in a cell viability assay, IC50 = 0.03469 μM. | SANGER | |||
| HH | Growth inhibition assay | Inhibition of human HH cell growth in a cell viability assay, IC50 = 0.03506 μM. | SANGER | |||
| CAL-27 | Growth inhibition assay | Inhibition of human CAL-27 cell growth in a cell viability assay, IC50 = 0.03516 μM. | SANGER | |||
| NEC8 | Growth inhibition assay | Inhibition of human NEC8 cell growth in a cell viability assay, IC50 = 0.03537 μM. | SANGER | |||
| BxPC-3 | Growth inhibition assay | Inhibition of human BxPC-3 cell growth in a cell viability assay, IC50 = 0.03691 μM. | SANGER | |||
| SNB75 | Growth inhibition assay | Inhibition of human SNB75 cell growth in a cell viability assay, IC50 = 0.03724 μM. | SANGER | |||
| NB13 | Growth inhibition assay | Inhibition of human NB13 cell growth in a cell viability assay, IC50 = 0.03823 μM. | SANGER | |||
| SK-OV-3 | Growth inhibition assay | Inhibition of human SK-OV-3 cell growth in a cell viability assay, IC50 = 0.03874 μM. | SANGER | |||
| ME-180 | Growth inhibition assay | Inhibition of human ME-180 cell growth in a cell viability assay, IC50 = 0.0388 μM. | SANGER | |||
| JiyoyeP-2003 | Growth inhibition assay | Inhibition of human JiyoyeP-2003 cell growth in a cell viability assay, IC50 = 0.03938 μM. | SANGER | |||
| LU-134-A | Growth inhibition assay | Inhibition of human LU-134-A cell growth in a cell viability assay, IC50 = 0.04002 μM. | SANGER | |||
| LS-123 | Growth inhibition assay | Inhibition of human LS-123 cell growth in a cell viability assay, IC50 = 0.04028 μM. | SANGER | |||
| COLO-800 | Growth inhibition assay | Inhibition of human COLO-800 cell growth in a cell viability assay, IC50 = 0.04056 μM. | SANGER | |||
| LB831-BLC | Growth inhibition assay | Inhibition of human LB831-BLC cell growth in a cell viability assay, IC50 = 0.04185 μM. | SANGER | |||
| NCI-H747 | Growth inhibition assay | Inhibition of human NCI-H747 cell growth in a cell viability assay, IC50 = 0.04228 μM. | SANGER | |||
| MZ7-mel | Growth inhibition assay | Inhibition of human MZ7-mel cell growth in a cell viability assay, IC50 = 0.04266 μM. | SANGER | |||
| GT3TKB | Growth inhibition assay | Inhibition of human GT3TKB cell growth in a cell viability assay, IC50 = 0.04272 μM. | SANGER | |||
| 23132-87 | Growth inhibition assay | Inhibition of human 23132-87 cell growth in a cell viability assay, IC50 = 0.04305 μM. | SANGER | |||
| MOLT-16 | Growth inhibition assay | Inhibition of human MOLT-16 cell growth in a cell viability assay, IC50 = 0.04305 μM. | SANGER | |||
| PF-382 | Growth inhibition assay | Inhibition of human PF-382 cell growth in a cell viability assay, IC50 = 0.04422 μM. | SANGER | |||
| ES3 | Growth inhibition assay | Inhibition of human ES3 cell growth in a cell viability assay, IC50 = 0.0446 μM. | SANGER | |||
| SW756 | Growth inhibition assay | Inhibition of human SW756 cell growth in a cell viability assay, IC50 = 0.04514 μM. | SANGER | |||
| OAW-28 | Growth inhibition assay | Inhibition of human OAW-28 cell growth in a cell viability assay, IC50 = 0.04536 μM. | SANGER | |||
| RPMI-8402 | Growth inhibition assay | Inhibition of human RPMI-8402 cell growth in a cell viability assay, IC50 = 0.04593 μM. | SANGER | |||
| NCI-H1693 | Growth inhibition assay | Inhibition of human NCI-H1693 cell growth in a cell viability assay, IC50 = 0.04609 μM. | SANGER | |||
| MS-1 | Growth inhibition assay | Inhibition of human MS-1 cell growth in a cell viability assay, IC50 = 0.04634 μM. | SANGER | |||
| WSU-NHL | Growth inhibition assay | Inhibition of human WSU-NHL cell growth in a cell viability assay, IC50 = 0.05035 μM. | SANGER | |||
| HCT-116 | Growth inhibition assay | Inhibition of human HCT-116 cell growth in a cell viability assay, IC50 = 0.05083 μM. | SANGER | |||
| SF295 | Growth inhibition assay | Inhibition of human SF295 cell growth in a cell viability assay, IC50 = 0.05112 μM. | SANGER | |||
| MFE-296 | Growth inhibition assay | Inhibition of human MFE-296 cell growth in a cell viability assay, IC50 = 0.05135 μM. | SANGER | |||
| NCI-H209 | Growth inhibition assay | Inhibition of human NCI-H209 cell growth in a cell viability assay, IC50 = 0.05207 μM. | SANGER | |||
| SW962 | Growth inhibition assay | Inhibition of human SW962 cell growth in a cell viability assay, IC50 = 0.05241 μM. | SANGER | |||
| CTB-1 | Growth inhibition assay | Inhibition of human CTB-1 cell growth in a cell viability assay, IC50 = 0.05339 μM. | SANGER | |||
| EFO-21 | Growth inhibition assay | Inhibition of human EFO-21 cell growth in a cell viability assay, IC50 = 0.05366 μM. | SANGER | |||
| A704 | Growth inhibition assay | Inhibition of human A704 cell growth in a cell viability assay, IC50 = 0.05378 μM. | SANGER | |||
| COR-L279 | Growth inhibition assay | Inhibition of human COR-L279 cell growth in a cell viability assay, IC50 = 0.05391 μM. | SANGER | |||
| HN | Growth inhibition assay | Inhibition of human HN cell growth in a cell viability assay, IC50 = 0.05409 μM. | SANGER | |||
| Caov-3 | Growth inhibition assay | Inhibition of human Caov-3 cell growth in a cell viability assay, IC50 = 0.05413 μM. | SANGER | |||
| NCI-H1770 | Growth inhibition assay | Inhibition of human NCI-H1770 cell growth in a cell viability assay, IC50 = 0.05504 μM. | SANGER | |||
| G-401 | Growth inhibition assay | Inhibition of human G-401 cell growth in a cell viability assay, IC50 = 0.05516 μM. | SANGER | |||
| KYSE-410 | Growth inhibition assay | Inhibition of human KYSE-410 cell growth in a cell viability assay, IC50 = 0.05587 μM. | SANGER | |||
| OE33 | Growth inhibition assay | Inhibition of human OE33 cell growth in a cell viability assay, IC50 = 0.06117 μM. | SANGER | |||
| NCI-H1694 | Growth inhibition assay | Inhibition of human NCI-H1694 cell growth in a cell viability assay, IC50 = 0.06129 μM. | SANGER | |||
| KG-1 | Growth inhibition assay | Inhibition of human KG-1 cell growth in a cell viability assay, IC50 = 0.0622 μM. | SANGER | |||
| SNU-423 | Growth inhibition assay | Inhibition of human SNU-423 cell growth in a cell viability assay, IC50 = 0.06248 μM. | SANGER | |||
| GDM-1 | Growth inhibition assay | Inhibition of human GDM-1 cell growth in a cell viability assay, IC50 = 0.06254 μM. | SANGER | |||
| SU-DHL-1 | Growth inhibition assay | Inhibition of human SU-DHL-1 cell growth in a cell viability assay, IC50 = 0.06266 μM. | SANGER | |||
| LB2518-MEL | Growth inhibition assay | Inhibition of human LB2518-MEL cell growth in a cell viability assay, IC50 = 0.06452 μM. | SANGER | |||
| LB996-RCC | Growth inhibition assay | Inhibition of human LB996-RCC cell growth in a cell viability assay, IC50 = 0.06509 μM. | SANGER | |||
| MOLT-4 | Growth inhibition assay | Inhibition of human MOLT-4 cell growth in a cell viability assay, IC50 = 0.06528 μM. | SANGER | |||
| J-RT3-T3-5 | Growth inhibition assay | Inhibition of human J-RT3-T3-5 cell growth in a cell viability assay, IC50 = 0.06718 μM. | SANGER | |||
| HCC1599 | Growth inhibition assay | Inhibition of human HCC1599 cell growth in a cell viability assay, IC50 = 0.07022 μM. | SANGER | |||
| TYK-nu | Growth inhibition assay | Inhibition of human TYK-nu cell growth in a cell viability assay, IC50 = 0.07264 μM. | SANGER | |||
| EW-18 | Growth inhibition assay | Inhibition of human EW-18 cell growth in a cell viability assay, IC50 = 0.07275 μM. | SANGER | |||
| LC4-1 | Growth inhibition assay | Inhibition of human LC4-1 cell growth in a cell viability assay, IC50 = 0.07474 μM. | SANGER | |||
| COLO-680N | Growth inhibition assay | Inhibition of human COLO-680N cell growth in a cell viability assay, IC50 = 0.07549 μM. | SANGER | |||
| MKN1 | Growth inhibition assay | Inhibition of human MKN1 cell growth in a cell viability assay, IC50 = 0.07837 μM. | SANGER | |||
| HCT-15 | Growth inhibition assay | Inhibition of human HCT-15 cell growth in a cell viability assay, IC50 = 0.08216 μM. | SANGER | |||
| NCI-H1882 | Growth inhibition assay | Inhibition of human NCI-H1882 cell growth in a cell viability assay, IC50 = 0.08245 μM. | SANGER | |||
| IMR-5 | Growth inhibition assay | Inhibition of human IMR-5 cell growth in a cell viability assay, IC50 = 0.08296 μM. | SANGER | |||
| DB | Growth inhibition assay | Inhibition of human DB cell growth in a cell viability assay, IC50 = 0.0844 μM. | SANGER | |||
| P12-ICHIKAWA | Growth inhibition assay | Inhibition of human P12-ICHIKAWA cell growth in a cell viability assay, IC50 = 0.0847 μM. | SANGER | |||
| KARPAS-422 | Growth inhibition assay | Inhibition of human KARPAS-422 cell growth in a cell viability assay, IC50 = 0.08579 μM. | SANGER | |||
| SK-N-DZ | Growth inhibition assay | Inhibition of human SK-N-DZ cell growth in a cell viability assay, IC50 = 0.08656 μM. | SANGER | |||
| FTC-133 | Growth inhibition assay | Inhibition of human FTC-133 cell growth in a cell viability assay, IC50 = 0.08749 μM. | SANGER | |||
| SCC-3 | Growth inhibition assay | Inhibition of human SCC-3 cell growth in a cell viability assay, IC50 = 0.08964 μM. | SANGER | |||
| KM12 | Growth inhibition assay | Inhibition of human KM12 cell growth in a cell viability assay, IC50 = 0.09149 μM. | SANGER | |||
| OAW-42 | Growth inhibition assay | Inhibition of human OAW-42 cell growth in a cell viability assay, IC50 = 0.09214 μM. | SANGER | |||
| GCIY | Growth inhibition assay | Inhibition of human GCIY cell growth in a cell viability assay, IC50 = 0.09269 μM. | SANGER | |||
| KYSE-520 | Growth inhibition assay | Inhibition of human KYSE-520 cell growth in a cell viability assay, IC50 = 0.09284 μM. | SANGER | |||
| RPMI-8866 | Growth inhibition assay | Inhibition of human RPMI-8866 cell growth in a cell viability assay, IC50 = 0.09523 μM. | SANGER | |||
| L-363 | Growth inhibition assay | Inhibition of human L-363 cell growth in a cell viability assay, IC50 = 0.0955 μM. | SANGER | |||
| 22RV1 | Growth inhibition assay | Inhibition of human 22RV1 cell growth in a cell viability assay, IC50 = 0.09648 μM. | SANGER | |||
| DSH1 | Growth inhibition assay | Inhibition of human DSH1 cell growth in a cell viability assay, IC50 = 0.0965 μM. | SANGER | |||
| A253 | Growth inhibition assay | Inhibition of human A253 cell growth in a cell viability assay, IC50 = 0.10228 μM. | SANGER | |||
| NCI-H661 | Growth inhibition assay | Inhibition of human NCI-H661 cell growth in a cell viability assay, IC50 = 0.10402 μM. | SANGER | |||
| SK-MEL-3 | Growth inhibition assay | Inhibition of human SK-MEL-3 cell growth in a cell viability assay, IC50 = 0.1051 μM. | SANGER | |||
| FADU | Growth inhibition assay | Inhibition of human FADU cell growth in a cell viability assay, IC50 = 0.10545 μM. | SANGER | |||
| SJRH30 | Growth inhibition assay | Inhibition of human SJRH30 cell growth in a cell viability assay, IC50 = 0.10641 μM. | SANGER | |||
| HCC1569 | Growth inhibition assay | Inhibition of human HCC1569 cell growth in a cell viability assay, IC50 = 0.10936 μM. | SANGER | |||
| NCI-H526 | Growth inhibition assay | Inhibition of human NCI-H526 cell growth in a cell viability assay, IC50 = 0.10988 μM. | SANGER | |||
| BL-70 | Growth inhibition assay | Inhibition of human BL-70 cell growth in a cell viability assay, IC50 = 0.11097 μM. | SANGER | |||
| SW1990 | Growth inhibition assay | Inhibition of human SW1990 cell growth in a cell viability assay, IC50 = 0.11307 μM. | SANGER | |||
| LAMA-84 | Growth inhibition assay | Inhibition of human LAMA-84 cell growth in a cell viability assay, IC50 = 0.11504 μM. | SANGER | |||
| COLO-741 | Growth inhibition assay | Inhibition of human COLO-741 cell growth in a cell viability assay, IC50 = 0.12012 μM. | SANGER | |||
| SCC-15 | Growth inhibition assay | Inhibition of human SCC-15 cell growth in a cell viability assay, IC50 = 0.12113 μM. | SANGER | |||
| DBTRG-05MG | Growth inhibition assay | Inhibition of human DBTRG-05MG cell growth in a cell viability assay, IC50 = 0.12182 μM. | SANGER | |||
| HEC-1 | Growth inhibition assay | Inhibition of human HEC-1 cell growth in a cell viability assay, IC50 = 0.12363 μM. | SANGER | |||
| D-283MED | Growth inhibition assay | Inhibition of human D-283MED cell growth in a cell viability assay, IC50 = 0.12698 μM. | SANGER | |||
| RD | Growth inhibition assay | Inhibition of human RD cell growth in a cell viability assay, IC50 = 0.13011 μM. | SANGER | |||
| K052 | Growth inhibition assay | Inhibition of human K052 cell growth in a cell viability assay, IC50 = 0.13671 μM. | SANGER | |||
| CAL-85-1 | Growth inhibition assay | Inhibition of human CAL-85-1 cell growth in a cell viability assay, IC50 = 0.14463 μM. | SANGER | |||
| NCI-H2052 | Growth inhibition assay | Inhibition of human NCI-H2052 cell growth in a cell viability assay, IC50 = 0.14482 μM. | SANGER | |||
| BFTC-909 | Growth inhibition assay | Inhibition of human BFTC-909 cell growth in a cell viability assay, IC50 = 0.1456 μM. | SANGER | |||
| HuP-T3 | Growth inhibition assay | Inhibition of human HuP-T3 cell growth in a cell viability assay, IC50 = 0.14565 μM. | SANGER | |||
| NCI-H64 | Growth inhibition assay | Inhibition of human NCI-H64 cell growth in a cell viability assay, IC50 = 0.15093 μM. | SANGER | |||
| C-4-II | Growth inhibition assay | Inhibition of human C-4-II cell growth in a cell viability assay, IC50 = 0.15237 μM. | SANGER | |||
| KMOE-2 | Growth inhibition assay | Inhibition of human KMOE-2 cell growth in a cell viability assay, IC50 = 0.15493 μM. | SANGER | |||
| NB12 | Growth inhibition assay | Inhibition of human NB12 cell growth in a cell viability assay, IC50 = 0.15515 μM. | SANGER | |||
| EM-2 | Growth inhibition assay | Inhibition of human EM-2 cell growth in a cell viability assay, IC50 = 0.1599 μM. | SANGER | |||
| SIMA | Growth inhibition assay | Inhibition of human SIMA cell growth in a cell viability assay, IC50 = 0.16125 μM. | SANGER | |||
| SBC-1 | Growth inhibition assay | Inhibition of human SBC-1 cell growth in a cell viability assay, IC50 = 0.16557 μM. | SANGER | |||
| KS-1 | Growth inhibition assay | Inhibition of human KS-1 cell growth in a cell viability assay, IC50 = 0.16652 μM. | SANGER | |||
| no-10 | Growth inhibition assay | Inhibition of human no-10 cell growth in a cell viability assay, IC50 = 0.17376 μM. | SANGER | |||
| NCCIT | Growth inhibition assay | Inhibition of human NCCIT cell growth in a cell viability assay, IC50 = 0.17626 μM. | SANGER | |||
| RERF-LC-MS | Growth inhibition assay | Inhibition of human RERF-LC-MS cell growth in a cell viability assay, IC50 = 0.17669 μM. | SANGER | |||
| BT-20 | Growth inhibition assay | Inhibition of human BT-20 cell growth in a cell viability assay, IC50 = 0.18174 μM. | SANGER | |||
| NCI-H1623 | Growth inhibition assay | Inhibition of human NCI-H1623 cell growth in a cell viability assay, IC50 = 0.18708 μM. | SANGER | |||
| TE-9 | Growth inhibition assay | Inhibition of human TE-9 cell growth in a cell viability assay, IC50 = 0.18963 μM. | SANGER | |||
| U-87-MG | Growth inhibition assay | Inhibition of human U-87-MG cell growth in a cell viability assay, IC50 = 0.19057 μM. | SANGER | |||
| CAL-51 | Growth inhibition assay | Inhibition of human CAL-51 cell growth in a cell viability assay, IC50 = 0.19114 μM. | SANGER | |||
| 639-V | Growth inhibition assay | Inhibition of human 639-V cell growth in a cell viability assay, IC50 = 0.19314 μM. | SANGER | |||
| SJSA-1 | Growth inhibition assay | Inhibition of human SJSA-1 cell growth in a cell viability assay, IC50 = 0.19501 μM. | SANGER | |||
| DOHH-2 | Growth inhibition assay | Inhibition of human DOHH-2 cell growth in a cell viability assay, IC50 = 0.19564 μM. | SANGER | |||
| IST-SL1 | Growth inhibition assay | Inhibition of human IST-SL1 cell growth in a cell viability assay, IC50 = 0.19747 μM. | SANGER | |||
| NCI-H1618 | Growth inhibition assay | Inhibition of human NCI-H1618 cell growth in a cell viability assay, IC50 = 0.19756 μM. | SANGER | |||
| TGW | Growth inhibition assay | Inhibition of human TGW cell growth in a cell viability assay, IC50 = 0.19964 μM. | SANGER | |||
| HT-3 | Growth inhibition assay | Inhibition of human HT-3 cell growth in a cell viability assay, IC50 = 0.20054 μM. | SANGER | |||
| AN3-CA | Growth inhibition assay | Inhibition of human AN3-CA cell growth in a cell viability assay, IC50 = 0.20329 μM. | SANGER | |||
| PC-14 | Growth inhibition assay | Inhibition of human PC-14 cell growth in a cell viability assay, IC50 = 0.20331 μM. | SANGER | |||
| BHT-101 | Growth inhibition assay | Inhibition of human BHT-101 cell growth in a cell viability assay, IC50 = 0.21039 μM. | SANGER | |||
| NCI-H23 | Growth inhibition assay | Inhibition of human NCI-H23 cell growth in a cell viability assay, IC50 = 0.21106 μM. | SANGER | |||
| SCC-4 | Growth inhibition assay | Inhibition of human SCC-4 cell growth in a cell viability assay, IC50 = 0.21185 μM. | SANGER | |||
| EGI-1 | Growth inhibition assay | Inhibition of human EGI-1 cell growth in a cell viability assay, IC50 = 0.21386 μM. | SANGER | |||
| Calu-1 | Growth inhibition assay | Inhibition of human Calu-1 cell growth in a cell viability assay, IC50 = 0.22003 μM. | SANGER | |||
| BC-3 | Growth inhibition assay | Inhibition of human BC-3 cell growth in a cell viability assay, IC50 = 0.22065 μM. | SANGER | |||
| HOP-62 | Growth inhibition assay | Inhibition of human HOP-62 cell growth in a cell viability assay, IC50 = 0.22258 μM. | SANGER | |||
| NCI-H1793 | Growth inhibition assay | Inhibition of human NCI-H1793 cell growth in a cell viability assay, IC50 = 0.22363 μM. | SANGER | |||
| COLO-320-HSR | Growth inhibition assay | Inhibition of human COLO-320-HSR cell growth in a cell viability assay, IC50 = 0.22408 μM. | SANGER | |||
| NCI-H596 | Growth inhibition assay | Inhibition of human NCI-H596 cell growth in a cell viability assay, IC50 = 0.22513 μM. | SANGER | |||
| EHEB | Growth inhibition assay | Inhibition of human EHEB cell growth in a cell viability assay, IC50 = 0.22651 μM. | SANGER | |||
| BEN | Growth inhibition assay | Inhibition of human BEN cell growth in a cell viability assay, IC50 = 0.23791 μM. | SANGER | |||
| MHH-PREB-1 | Growth inhibition assay | Inhibition of human MHH-PREB-1 cell growth in a cell viability assay, IC50 = 0.24804 μM. | SANGER | |||
| TE-6 | Growth inhibition assay | Inhibition of human TE-6 cell growth in a cell viability assay, IC50 = 0.25035 μM. | SANGER | |||
| KARPAS-299 | Growth inhibition assay | Inhibition of human KARPAS-299 cell growth in a cell viability assay, IC50 = 0.252 μM. | SANGER | |||
| BOKU | Growth inhibition assay | Inhibition of human BOKU cell growth in a cell viability assay, IC50 = 0.25433 μM. | SANGER | |||
| MZ1-PC | Growth inhibition assay | Inhibition of human MZ1-PC cell growth in a cell viability assay, IC50 = 0.25435 μM. | SANGER | |||
| IPC-298 | Growth inhibition assay | Inhibition of human IPC-298 cell growth in a cell viability assay, IC50 = 0.25477 μM. | SANGER | |||
| NCI-H1792 | Growth inhibition assay | Inhibition of human NCI-H1792 cell growth in a cell viability assay, IC50 = 0.25904 μM. | SANGER | |||
| KM-H2 | Growth inhibition assay | Inhibition of human KM-H2 cell growth in a cell viability assay, IC50 = 0.26068 μM. | SANGER | |||
| Becker | Growth inhibition assay | Inhibition of human Becker cell growth in a cell viability assay, IC50 = 0.26704 μM. | SANGER | |||
| NCI-H446 | Growth inhibition assay | Inhibition of human NCI-H446 cell growth in a cell viability assay, IC50 = 0.26911 μM. | SANGER | |||
| MLMA | Growth inhibition assay | Inhibition of human MLMA cell growth in a cell viability assay, IC50 = 0.27156 μM. | SANGER | |||
| JEG-3 | Growth inhibition assay | Inhibition of human JEG-3 cell growth in a cell viability assay, IC50 = 0.27669 μM. | SANGER | |||
| SCC-25 | Growth inhibition assay | Inhibition of human SCC-25 cell growth in a cell viability assay, IC50 = 0.28928 μM. | SANGER | |||
| CA46 | Growth inhibition assay | Inhibition of human CA46 cell growth in a cell viability assay, IC50 = 0.29339 μM. | SANGER | |||
| CAL-54 | Growth inhibition assay | Inhibition of human CAL-54 cell growth in a cell viability assay, IC50 = 0.29759 μM. | SANGER | |||
| KYSE-70 | Growth inhibition assay | Inhibition of human KYSE-70 cell growth in a cell viability assay, IC50 = 0.29969 μM. | SANGER | |||
| LU-65 | Growth inhibition assay | Inhibition of human LU-65 cell growth in a cell viability assay, IC50 = 0.30381 μM. | SANGER | |||
| OVCAR-5 | Growth inhibition assay | Inhibition of human OVCAR-5 cell growth in a cell viability assay, IC50 = 0.30577 μM. | SANGER | |||
| NCI-H2081 | Growth inhibition assay | Inhibition of human NCI-H2081 cell growth in a cell viability assay, IC50 = 0.31077 μM. | SANGER | |||
| NCI-H226 | Growth inhibition assay | Inhibition of human NCI-H226 cell growth in a cell viability assay, IC50 = 0.31696 μM. | SANGER | |||
| A427 | Growth inhibition assay | Inhibition of human A427 cell growth in a cell viability assay, IC50 = 0.32398 μM. | SANGER | |||
| CPC-N | Growth inhibition assay | Inhibition of human CPC-N cell growth in a cell viability assay, IC50 = 0.329 μM. | SANGER | |||
| SW13 | Growth inhibition assay | Inhibition of human SW13 cell growth in a cell viability assay, IC50 = 0.33037 μM. | SANGER | |||
| K-562 | Growth inhibition assay | Inhibition of human K-562 cell growth in a cell viability assay, IC50 = 0.33298 μM. | SANGER | |||
| NCI-N87 | Growth inhibition assay | Inhibition of human NCI-N87 cell growth in a cell viability assay, IC50 = 0.33304 μM. | SANGER | |||
| U-698-M | Growth inhibition assay | Inhibition of human U-698-M cell growth in a cell viability assay, IC50 = 0.34453 μM. | SANGER | |||
| IM-9 | Growth inhibition assay | Inhibition of human IM-9 cell growth in a cell viability assay, IC50 = 0.34691 μM. | SANGER | |||
| NCI-H748 | Growth inhibition assay | Inhibition of human NCI-H748 cell growth in a cell viability assay, IC50 = 0.35458 μM. | SANGER | |||
| UACC-257 | Growth inhibition assay | Inhibition of human UACC-257 cell growth in a cell viability assay, IC50 = 0.36412 μM. | SANGER | |||
| HT-1376 | Growth inhibition assay | Inhibition of human HT-1376 cell growth in a cell viability assay, IC50 = 0.36895 μM. | SANGER | |||
| GAK | Growth inhibition assay | Inhibition of human GAK cell growth in a cell viability assay, IC50 = 0.37294 μM. | SANGER | |||
| NCI-H82 | Growth inhibition assay | Inhibition of human NCI-H82 cell growth in a cell viability assay, IC50 = 0.37297 μM. | SANGER | |||
| NCI-H1304 | Growth inhibition assay | Inhibition of human NCI-H1304 cell growth in a cell viability assay, IC50 = 0.38454 μM. | SANGER | |||
| MHH-NB-11 | Growth inhibition assay | Inhibition of human MHH-NB-11 cell growth in a cell viability assay, IC50 = 0.38597 μM. | SANGER | |||
| CAMA-1 | Growth inhibition assay | Inhibition of human CAMA-1 cell growth in a cell viability assay, IC50 = 0.3958 μM. | SANGER | |||
| GCT | Growth inhibition assay | Inhibition of human GCT cell growth in a cell viability assay, IC50 = 0.4051 μM. | SANGER | |||
| HPAF-II | Growth inhibition assay | Inhibition of human HPAF-II cell growth in a cell viability assay, IC50 = 0.42847 μM. | SANGER | |||
| Raji | Growth inhibition assay | Inhibition of human Raji cell growth in a cell viability assay, IC50 = 0.43145 μM. | SANGER | |||
| EW-11 | Growth inhibition assay | Inhibition of human EW-11 cell growth in a cell viability assay, IC50 = 0.43352 μM. | SANGER | |||
| SW1573 | Growth inhibition assay | Inhibition of human SW1573 cell growth in a cell viability assay, IC50 = 0.45733 μM. | SANGER | |||
| KLE | Growth inhibition assay | Inhibition of human KLE cell growth in a cell viability assay, IC50 = 0.45942 μM. | SANGER | |||
| NCI-H69 | Growth inhibition assay | Inhibition of human NCI-H69 cell growth in a cell viability assay, IC50 = 0.45948 μM. | SANGER | |||
| MDA-MB-361 | Growth inhibition assay | Inhibition of human MDA-MB-361 cell growth in a cell viability assay, IC50 = 0.46064 μM. | SANGER | |||
| SW48 | Growth inhibition assay | Inhibition of human SW48 cell growth in a cell viability assay, IC50 = 0.46259 μM. | SANGER | |||
| SK-MM-2 | Growth inhibition assay | Inhibition of human SK-MM-2 cell growth in a cell viability assay, IC50 = 0.47912 μM. | SANGER | |||
| MC116 | Growth inhibition assay | Inhibition of human MC116 cell growth in a cell viability assay, IC50 = 0.48166 μM. | SANGER | |||
| NB1 | Growth inhibition assay | Inhibition of human NB1 cell growth in a cell viability assay, IC50 = 0.48753 μM. | SANGER | |||
| NCI-H1155 | Growth inhibition assay | Inhibition of human NCI-H1155 cell growth in a cell viability assay, IC50 = 0.48828 μM. | SANGER | |||
| SN12C | Growth inhibition assay | Inhibition of human SN12C cell growth in a cell viability assay, IC50 = 0.49734 μM. | SANGER | |||
| NCI-H838 | Growth inhibition assay | Inhibition of human NCI-H838 cell growth in a cell viability assay, IC50 = 0.49875 μM. | SANGER | |||
| SW1463 | Growth inhibition assay | Inhibition of human SW1463 cell growth in a cell viability assay, IC50 = 0.51017 μM. | SANGER | |||
| NCI-H1648 | Growth inhibition assay | Inhibition of human NCI-H1648 cell growth in a cell viability assay, IC50 = 0.51081 μM. | SANGER | |||
| M14 | Growth inhibition assay | Inhibition of human M14 cell growth in a cell viability assay, IC50 = 0.51466 μM. | SANGER | |||
| T98G | Growth inhibition assay | Inhibition of human T98G cell growth in a cell viability assay, IC50 = 0.53948 μM. | SANGER | |||
| CaR-1 | Growth inhibition assay | Inhibition of human CaR-1 cell growth in a cell viability assay, IC50 = 0.55122 μM. | SANGER | |||
| NCI-H650 | Growth inhibition assay | Inhibition of human NCI-H650 cell growth in a cell viability assay, IC50 = 0.56569 μM. | SANGER | |||
| HuH-7 | Growth inhibition assay | Inhibition of human HuH-7 cell growth in a cell viability assay, IC50 = 0.56861 μM. | SANGER | |||
| Daudi | Growth inhibition assay | Inhibition of human Daudi cell growth in a cell viability assay, IC50 = 0.56949 μM. | SANGER | |||
| CAL-120 | Growth inhibition assay | Inhibition of human CAL-120 cell growth in a cell viability assay, IC50 = 0.57588 μM. | SANGER | |||
| EW-3 | Growth inhibition assay | Inhibition of human EW-3 cell growth in a cell viability assay, IC50 = 0.57956 μM. | SANGER | |||
| OMC-1 | Growth inhibition assay | Inhibition of human OMC-1 cell growth in a cell viability assay, IC50 = 0.58333 μM. | SANGER | |||
| U-266 | Growth inhibition assay | Inhibition of human U-266 cell growth in a cell viability assay, IC50 = 0.60794 μM. | SANGER | |||
| OVCAR-4 | Growth inhibition assay | Inhibition of human OVCAR-4 cell growth in a cell viability assay, IC50 = 0.62523 μM. | SANGER | |||
| RCC10RGB | Growth inhibition assay | Inhibition of human RCC10RGB cell growth in a cell viability assay, IC50 = 0.63374 μM. | SANGER | |||
| NCI-H2141 | Growth inhibition assay | Inhibition of human NCI-H2141 cell growth in a cell viability assay, IC50 = 0.64404 μM. | SANGER | |||
| Ramos-2G6-4C10 | Growth inhibition assay | Inhibition of human Ramos-2G6-4C10 cell growth in a cell viability assay, IC50 = 0.66087 μM. | SANGER | |||
| THP-1 | Growth inhibition assay | Inhibition of human THP-1 cell growth in a cell viability assay, IC50 = 0.6642 μM. | SANGER | |||
| RCM-1 | Growth inhibition assay | Inhibition of human RCM-1 cell growth in a cell viability assay, IC50 = 0.66478 μM. | SANGER | |||
| K5 | Growth inhibition assay | Inhibition of human K5 cell growth in a cell viability assay, IC50 = 0.68269 μM. | SANGER | |||
| MPP-89 | Growth inhibition assay | Inhibition of human MPP-89 cell growth in a cell viability assay, IC50 = 0.69228 μM. | SANGER | |||
| ChaGo-K-1 | Growth inhibition assay | Inhibition of human ChaGo-K-1 cell growth in a cell viability assay, IC50 = 0.6956 μM. | SANGER | |||
| OE19 | Growth inhibition assay | Inhibition of human OE19 cell growth in a cell viability assay, IC50 = 0.70216 μM. | SANGER | |||
| NCI-H1755 | Growth inhibition assay | Inhibition of human NCI-H1755 cell growth in a cell viability assay, IC50 = 0.71816 μM. | SANGER | |||
| KNS-42 | Growth inhibition assay | Inhibition of human KNS-42 cell growth in a cell viability assay, IC50 = 0.73582 μM. | SANGER | |||
| no-11 | Growth inhibition assay | Inhibition of human no-11 cell growth in a cell viability assay, IC50 = 0.73668 μM. | SANGER | |||
| IST-MES1 | Growth inhibition assay | Inhibition of human IST-MES1 cell growth in a cell viability assay, IC50 = 0.77354 μM. | SANGER | |||
| NCI-H2347 | Growth inhibition assay | Inhibition of human NCI-H2347 cell growth in a cell viability assay, IC50 = 0.79201 μM. | SANGER | |||
| SKG-IIIa | Growth inhibition assay | Inhibition of human SKG-IIIa cell growth in a cell viability assay, IC50 = 0.80972 μM. | SANGER | |||
| UACC-62 | Growth inhibition assay | Inhibition of human UACC-62 cell growth in a cell viability assay, IC50 = 0.8126 μM. | SANGER | |||
| SNU-387 | Growth inhibition assay | Inhibition of human SNU-387 cell growth in a cell viability assay, IC50 = 0.82744 μM. | SANGER | |||
| LS-513 | Growth inhibition assay | Inhibition of human LS-513 cell growth in a cell viability assay, IC50 = 0.88761 μM. | SANGER | |||
| NCI-H719 | Growth inhibition assay | Inhibition of human NCI-H719 cell growth in a cell viability assay, IC50 = 0.89157 μM. | SANGER | |||
| HOP-92 | Growth inhibition assay | Inhibition of human HOP-92 cell growth in a cell viability assay, IC50 = 0.95075 μM. | SANGER | |||
| CAS-1 | Growth inhibition assay | Inhibition of human CAS-1 cell growth in a cell viability assay, IC50 = 0.95508 μM. | SANGER | |||
| HTC-C3 | Growth inhibition assay | Inhibition of human HTC-C3 cell growth in a cell viability assay, IC50 = 0.9965 μM. | SANGER | |||
| D-392MG | Growth inhibition assay | Inhibition of human D-392MG cell growth in a cell viability assay, IC50 = 1.02288 μM. | SANGER | |||
| MHH-CALL-2 | Growth inhibition assay | Inhibition of human MHH-CALL-2 cell growth in a cell viability assay, IC50 = 1.02319 μM. | SANGER | |||
| DMS-53 | Growth inhibition assay | Inhibition of human DMS-53 cell growth in a cell viability assay, IC50 = 1.03815 μM. | SANGER | |||
| TGBC24TKB | Growth inhibition assay | Inhibition of human TGBC24TKB cell growth in a cell viability assay, IC50 = 1.04183 μM. | SANGER | |||
| NCI-H1417 | Growth inhibition assay | Inhibition of human NCI-H1417 cell growth in a cell viability assay, IC50 = 1.07075 μM. | SANGER | |||
| OVCAR-3 | Growth inhibition assay | Inhibition of human OVCAR-3 cell growth in a cell viability assay, IC50 = 1.0739 μM. | SANGER | |||
| RXF393 | Growth inhibition assay | Inhibition of human RXF393 cell growth in a cell viability assay, IC50 = 1.1432 μM. | SANGER | |||
| MKN28 | Growth inhibition assay | Inhibition of human MKN28 cell growth in a cell viability assay, IC50 = 1.15124 μM. | SANGER | |||
| MSTO-211H | Growth inhibition assay | Inhibition of human MSTO-211H cell growth in a cell viability assay, IC50 = 1.15257 μM. | SANGER | |||
| NCI-H2126 | Growth inhibition assay | Inhibition of human NCI-H2126 cell growth in a cell viability assay, IC50 = 1.15799 μM. | SANGER | |||
| TCCSUP | Growth inhibition assay | Inhibition of human TCCSUP cell growth in a cell viability assay, IC50 = 1.16785 μM. | SANGER | |||
| TE-12 | Growth inhibition assay | Inhibition of human TE-12 cell growth in a cell viability assay, IC50 = 1.17366 μM. | SANGER | |||
| NCI-H1581 | Growth inhibition assay | Inhibition of human NCI-H1581 cell growth in a cell viability assay, IC50 = 1.17372 μM. | SANGER | |||
| GOTO | Growth inhibition assay | Inhibition of human GOTO cell growth in a cell viability assay, IC50 = 1.20277 μM. | SANGER | |||
| NCI-H28 | Growth inhibition assay | Inhibition of human NCI-H28 cell growth in a cell viability assay, IC50 = 1.2149 μM. | SANGER | |||
| KNS-81-FD | Growth inhibition assay | Inhibition of human KNS-81-FD cell growth in a cell viability assay, IC50 = 1.23463 μM. | SANGER | |||
| YT | Growth inhibition assay | Inhibition of human YT cell growth in a cell viability assay, IC50 = 1.28559 μM. | SANGER | |||
| NB5 | Growth inhibition assay | Inhibition of human NB5 cell growth in a cell viability assay, IC50 = 1.32585 μM. | SANGER | |||
| U-118-MG | Growth inhibition assay | Inhibition of human U-118-MG cell growth in a cell viability assay, IC50 = 1.35159 μM. | SANGER | |||
| LS-1034 | Growth inhibition assay | Inhibition of human LS-1034 cell growth in a cell viability assay, IC50 = 1.3845 μM. | SANGER | |||
| PANC-08-13 | Growth inhibition assay | Inhibition of human PANC-08-13 cell growth in a cell viability assay, IC50 = 1.39613 μM. | SANGER | |||
| COLO-205 | Growth inhibition assay | Inhibition of human COLO-205 cell growth in a cell viability assay, IC50 = 1.47181 μM. | SANGER | |||
| KURAMOCHI | Growth inhibition assay | Inhibition of human KURAMOCHI cell growth in a cell viability assay, IC50 = 1.49739 μM. | SANGER | |||
| SNU-C2B | Growth inhibition assay | Inhibition of human SNU-C2B cell growth in a cell viability assay, IC50 = 1.54777 μM. | SANGER | |||
| HDLM-2 | Growth inhibition assay | Inhibition of human HDLM-2 cell growth in a cell viability assay, IC50 = 1.63327 μM. | SANGER | |||
| PFSK-1 | Growth inhibition assay | Inhibition of human PFSK-1 cell growth in a cell viability assay, IC50 = 1.64794 μM. | SANGER | |||
| SW1088 | Growth inhibition assay | Inhibition of human SW1088 cell growth in a cell viability assay, IC50 = 1.66167 μM. | SANGER | |||
| LB373-MEL-D | Growth inhibition assay | Inhibition of human LB373-MEL-D cell growth in a cell viability assay, IC50 = 1.66495 μM. | SANGER | |||
| HT-1197 | Growth inhibition assay | Inhibition of human HT-1197 cell growth in a cell viability assay, IC50 = 1.76425 μM. | SANGER | |||
| MMAC-SF | Growth inhibition assay | Inhibition of human MMAC-SF cell growth in a cell viability assay, IC50 = 1.7766 μM. | SANGER | |||
| T-24 | Growth inhibition assay | Inhibition of human T-24 cell growth in a cell viability assay, IC50 = 2.07629 μM. | SANGER | |||
| LK-2 | Growth inhibition assay | Inhibition of human LK-2 cell growth in a cell viability assay, IC50 = 2.08563 μM. | SANGER | |||
| 5637 | Growth inhibition assay | Inhibition of human 5637 cell growth in a cell viability assay, IC50 = 2.10298 μM. | SANGER | |||
| GI-ME-N | Growth inhibition assay | Inhibition of human GI-ME-N cell growth in a cell viability assay, IC50 = 2.10851 μM. | SANGER | |||
| NCI-H2196 | Growth inhibition assay | Inhibition of human NCI-H2196 cell growth in a cell viability assay, IC50 = 2.31034 μM. | SANGER | |||
| KOSC-2 | Growth inhibition assay | Inhibition of human KOSC-2 cell growth in a cell viability assay, IC50 = 2.35338 μM. | SANGER | |||
| MN-60 | Growth inhibition assay | Inhibition of human MN-60 cell growth in a cell viability assay, IC50 = 2.43457 μM. | SANGER | |||
| AsPC-1 | Growth inhibition assay | Inhibition of human AsPC-1 cell growth in a cell viability assay, IC50 = 2.50301 μM. | SANGER | |||
| MDA-MB-175-VII | Growth inhibition assay | Inhibition of human MDA-MB-175-VII cell growth in a cell viability assay, IC50 = 2.51493 μM. | SANGER | |||
| DG-75 | Growth inhibition assay | Inhibition of human DG-75 cell growth in a cell viability assay, IC50 = 2.5612 μM. | SANGER | |||
| LNCaP-Clone-FGC | Growth inhibition assay | Inhibition of human LNCaP-Clone-FGC cell growth in a cell viability assay, IC50 = 2.65415 μM. | SANGER | |||
| SCLC-21H | Growth inhibition assay | Inhibition of human SCLC-21H cell growth in a cell viability assay, IC50 = 2.77414 μM. | SANGER | |||
| EFE-184 | Growth inhibition assay | Inhibition of human EFE-184 cell growth in a cell viability assay, IC50 = 2.79042 μM. | SANGER | |||
| HCC2157 | Growth inhibition assay | Inhibition of human HCC2157 cell growth in a cell viability assay, IC50 = 2.80678 μM. | SANGER | |||
| NCI-H1573 | Growth inhibition assay | Inhibition of human NCI-H1573 cell growth in a cell viability assay, IC50 = 2.80723 μM. | SANGER | |||
| PC-3 | Growth inhibition assay | Inhibition of human PC-3 cell growth in a cell viability assay, IC50 = 2.83163 μM. | SANGER | |||
| KY821 | Growth inhibition assay | Inhibition of human KY821 cell growth in a cell viability assay, IC50 = 2.8814 μM. | SANGER | |||
| ECC4 | Growth inhibition assay | Inhibition of human ECC4 cell growth in a cell viability assay, IC50 = 2.92765 μM. | SANGER | |||
| SK-N-AS | Growth inhibition assay | Inhibition of human SK-N-AS cell growth in a cell viability assay, IC50 = 2.96758 μM. | SANGER | |||
| NB6 | Growth inhibition assay | Inhibition of human NB6 cell growth in a cell viability assay, IC50 = 3.2819 μM. | SANGER | |||
| KMS-12-PE | Growth inhibition assay | Inhibition of human KMS-12-PE cell growth in a cell viability assay, IC50 = 3.55998 μM. | SANGER | |||
| NCI-H2171 | Growth inhibition assay | Inhibition of human NCI-H2171 cell growth in a cell viability assay, IC50 = 3.76535 μM. | SANGER | |||
| TE-11 | Growth inhibition assay | Inhibition of human TE-11 cell growth in a cell viability assay, IC50 = 4.09997 μM. | SANGER | |||
| DMS-153 | Growth inhibition assay | Inhibition of human DMS-153 cell growth in a cell viability assay, IC50 = 4.10246 μM. | SANGER | |||
| RVH-421 | Growth inhibition assay | Inhibition of human RVH-421 cell growth in a cell viability assay, IC50 = 4.11559 μM. | SANGER | |||
| RO82-W-1 | Growth inhibition assay | Inhibition of human RO82-W-1 cell growth in a cell viability assay, IC50 = 4.42356 μM. | SANGER | |||
| TE-1 | Growth inhibition assay | Inhibition of human TE-1 cell growth in a cell viability assay, IC50 = 5.86741 μM. | SANGER | |||
| MFE-280 | Growth inhibition assay | Inhibition of human MFE-280 cell growth in a cell viability assay, IC50 = 5.90388 μM. | SANGER | |||
| HT | Growth inhibition assay | Inhibition of human HT cell growth in a cell viability assay, IC50 = 5.93153 μM. | SANGER | |||
| NCI-H1963 | Growth inhibition assay | Inhibition of human NCI-H1963 cell growth in a cell viability assay, IC50 = 6.26713 μM. | SANGER | |||
| S-117 | Growth inhibition assay | Inhibition of human S-117 cell growth in a cell viability assay, IC50 = 6.30327 μM. | SANGER | |||
| TGBC1TKB | Growth inhibition assay | Inhibition of human TGBC1TKB cell growth in a cell viability assay, IC50 = 6.51712 μM. | SANGER | |||
| NCI-H1522 | Growth inhibition assay | Inhibition of human NCI-H1522 cell growth in a cell viability assay, IC50 = 6.53336 μM. | SANGER | |||
| TE-441-T | Growth inhibition assay | Inhibition of human TE-441-T cell growth in a cell viability assay, IC50 = 6.5501 μM. | SANGER | |||
| UACC-893 | Growth inhibition assay | Inhibition of human UACC-893 cell growth in a cell viability assay, IC50 = 6.55203 μM. | SANGER | |||
| SHP-77 | Growth inhibition assay | Inhibition of human SHP-77 cell growth in a cell viability assay, IC50 = 6.85463 μM. | SANGER | |||
| TALL-1 | Growth inhibition assay | Inhibition of human TALL-1 cell growth in a cell viability assay, IC50 = 7.00001 μM. | SANGER | |||
| T47D | Growth inhibition assay | Inhibition of human T47D cell growth in a cell viability assay, IC50 = 7.00094 μM. | SANGER | |||
| Capan-2 | Growth inhibition assay | Inhibition of human Capan-2 cell growth in a cell viability assay, IC50 = 7.09987 μM. | SANGER | |||
| SK-MEL-2 | Growth inhibition assay | Inhibition of human SK-MEL-2 cell growth in a cell viability assay, IC50 = 7.13094 μM. | SANGER | |||
| NCI-H1092 | Growth inhibition assay | Inhibition of human NCI-H1092 cell growth in a cell viability assay, IC50 = 7.15535 μM. | SANGER | |||
| LP-1 | Growth inhibition assay | Inhibition of human LP-1 cell growth in a cell viability assay, IC50 = 7.30969 μM. | SANGER | |||
| NCI-H889 | Growth inhibition assay | Inhibition of human NCI-H889 cell growth in a cell viability assay, IC50 = 7.57024 μM. | SANGER | |||
| NCI-H2452 | Growth inhibition assay | Inhibition of human NCI-H2452 cell growth in a cell viability assay, IC50 = 7.63456 μM. | SANGER | |||
| UMC-11 | Growth inhibition assay | Inhibition of human UMC-11 cell growth in a cell viability assay, IC50 = 8.35939 μM. | SANGER | |||
| LU-165 | Growth inhibition assay | Inhibition of human LU-165 cell growth in a cell viability assay, IC50 = 8.41085 μM. | SANGER | |||
| Mewo | Growth inhibition assay | Inhibition of human Mewo cell growth in a cell viability assay, IC50 = 8.43715 μM. | SANGER | |||
| C32 | Growth inhibition assay | Inhibition of human C32 cell growth in a cell viability assay, IC50 = 8.43927 μM. | SANGER | |||
| DV-90 | Growth inhibition assay | Inhibition of human DV-90 cell growth in a cell viability assay, IC50 = 8.45559 μM. | SANGER | |||
| SW1417 | Growth inhibition assay | Inhibition of human SW1417 cell growth in a cell viability assay, IC50 = 8.63434 μM. | SANGER | |||
| NCI-H187 | Growth inhibition assay | Inhibition of human NCI-H187 cell growth in a cell viability assay, IC50 = 8.85234 μM. | SANGER | |||
| LU-99A | Growth inhibition assay | Inhibition of human LU-99A cell growth in a cell viability assay, IC50 = 9.13742 μM. | SANGER | |||
| DMS-79 | Growth inhibition assay | Inhibition of human DMS-79 cell growth in a cell viability assay, IC50 = 9.4013 μM. | SANGER | |||
| MDA-MB-415 | Growth inhibition assay | Inhibition of human MDA-MB-415 cell growth in a cell viability assay, IC50 = 9.95336 μM. | SANGER | |||
| HCC1954 | Growth inhibition assay | Inhibition of human HCC1954 cell growth in a cell viability assay, IC50 = 9.97404 μM. | SANGER | |||
| EB-3 | Growth inhibition assay | Inhibition of human EB-3 cell growth in a cell viability assay, IC50 = 11.1641 μM. | SANGER | |||
| CW-2 | Growth inhibition assay | Inhibition of human CW-2 cell growth in a cell viability assay, IC50 = 11.2237 μM. | SANGER | |||
| COR-L88 | Growth inhibition assay | Inhibition of human COR-L88 cell growth in a cell viability assay, IC50 = 11.4887 μM. | SANGER | |||
| CP50-MEL-B | Growth inhibition assay | Inhibition of human CP50-MEL-B cell growth in a cell viability assay, IC50 = 11.567 μM. | SANGER | |||
| LN-405 | Growth inhibition assay | Inhibition of human LN-405 cell growth in a cell viability assay, IC50 = 11.8448 μM. | SANGER | |||
| EVSA-T | Growth inhibition assay | Inhibition of human EVSA-T cell growth in a cell viability assay, IC50 = 11.9609 μM. | SANGER | |||
| HCC70 | Growth inhibition assay | Inhibition of human HCC70 cell growth in a cell viability assay, IC50 = 12.0511 μM. | SANGER | |||
| UACC-812 | Growth inhibition assay | Inhibition of human UACC-812 cell growth in a cell viability assay, IC50 = 13.3687 μM. | SANGER | |||
| LC-1F | Growth inhibition assay | Inhibition of human LC-1F cell growth in a cell viability assay, IC50 = 13.4575 μM. | SANGER | |||
| HCC1419 | Growth inhibition assay | Inhibition of human HCC1419 cell growth in a cell viability assay, IC50 = 13.5715 μM. | SANGER | |||
| C2BBe1 | Growth inhibition assay | Inhibition of human C2BBe1 cell growth in a cell viability assay, IC50 = 13.6612 μM. | SANGER | |||
| COLO-678 | Growth inhibition assay | Inhibition of human COLO-678 cell growth in a cell viability assay, IC50 = 13.7484 μM. | SANGER | |||
| RT4 | Growth inhibition assay | Inhibition of human RT4 cell growth in a cell viability assay, IC50 = 13.8428 μM. | SANGER | |||
| NCI-H524 | Growth inhibition assay | Inhibition of human NCI-H524 cell growth in a cell viability assay, IC50 = 15.3769 μM. | SANGER | |||
| HCC1143 | Growth inhibition assay | Inhibition of human HCC1143 cell growth in a cell viability assay, IC50 = 16.3541 μM. | SANGER | |||
| HT55 | Growth inhibition assay | Inhibition of human HT55 cell growth in a cell viability assay, IC50 = 16.4103 μM. | SANGER | |||
| CAL-39 | Growth inhibition assay | Inhibition of human CAL-39 cell growth in a cell viability assay, IC50 = 16.6227 μM. | SANGER | |||
| KU-19-19 | Growth inhibition assay | Inhibition of human KU-19-19 cell growth in a cell viability assay, IC50 = 16.8123 μM. | SANGER | |||
| LB771-HNC | Growth inhibition assay | Inhibition of human LB771-HNC cell growth in a cell viability assay, IC50 = 16.9876 μM. | SANGER | |||
| OPM-2 | Growth inhibition assay | Inhibition of human OPM-2 cell growth in a cell viability assay, IC50 = 17.6337 μM. | SANGER | |||
| CAKI-1 | Growth inhibition assay | Inhibition of human CAKI-1 cell growth in a cell viability assay, IC50 = 18.0406 μM. | SANGER | |||
| KP-N-YN | Growth inhibition assay | Inhibition of human KP-N-YN cell growth in a cell viability assay, IC50 = 18.8885 μM. | SANGER | |||
| SW948 | Growth inhibition assay | Inhibition of human SW948 cell growth in a cell viability assay, IC50 = 20.4975 μM. | SANGER | |||
| SK-MEL-1 | Growth inhibition assay | Inhibition of human SK-MEL-1 cell growth in a cell viability assay, IC50 = 20.6083 μM. | SANGER | |||
| NCI-H1563 | Growth inhibition assay | Inhibition of human NCI-H1563 cell growth in a cell viability assay, IC50 = 21.5347 μM. | SANGER | |||
| GMS-10 | Growth inhibition assay | Inhibition of human GMS-10 cell growth in a cell viability assay, IC50 = 21.6061 μM. | SANGER | |||
| MDA-MB-453 | Growth inhibition assay | Inhibition of human MDA-MB-453 cell growth in a cell viability assay, IC50 = 22.2184 μM. | SANGER | |||
| PLC-PRF-5 | Growth inhibition assay | Inhibition of human PLC-PRF-5 cell growth in a cell viability assay, IC50 = 22.8813 μM. | SANGER | |||
| EFM-19 | Growth inhibition assay | Inhibition of human EFM-19 cell growth in a cell viability assay, IC50 = 24.2029 μM. | SANGER | |||
| SK-N-FI | Growth inhibition assay | Inhibition of human SK-N-FI cell growth in a cell viability assay, IC50 = 25.1429 μM. | SANGER | |||
| Saos-2 | Growth inhibition assay | Inhibition of human Saos-2 cell growth in a cell viability assay, IC50 = 26.765 μM. | SANGER | |||
| KARPAS-45 | Growth inhibition assay | Inhibition of human KARPAS-45 cell growth in a cell viability assay, IC50 = 27.5648 μM. | SANGER | |||
| EKVX | Growth inhibition assay | Inhibition of human EKVX cell growth in a cell viability assay, IC50 = 27.7875 μM. | SANGER | |||
| KINGS-1 | Growth inhibition assay | Inhibition of human KINGS-1 cell growth in a cell viability assay, IC50 = 30.879 μM. | SANGER | |||
| NCI-H2227 | Growth inhibition assay | Inhibition of human NCI-H2227 cell growth in a cell viability assay, IC50 = 31.2813 μM. | SANGER | |||
| D-542MG | Growth inhibition assay | Inhibition of human D-542MG cell growth in a cell viability assay, IC50 = 32.2968 μM. | SANGER | |||
| D-263MG | Growth inhibition assay | Inhibition of human D-263MG cell growth in a cell viability assay, IC50 = 33.5726 μM. | SANGER | |||
| A498 | Growth inhibition assay | Inhibition of human A498 cell growth in a cell viability assay, IC50 = 38.4491 μM. | SANGER | |||
| MDA-MB-157 | Growth inhibition assay | Inhibition of human MDA-MB-157 cell growth in a cell viability assay, IC50 = 38.5533 μM. | SANGER | |||
| RMG-I | Growth inhibition assay | Inhibition of human RMG-I cell growth in a cell viability assay, IC50 = 42.9009 μM. | SANGER | |||
| COLO-792 | Growth inhibition assay | Inhibition of human COLO-792 cell growth in a cell viability assay, IC50 = 43.2931 μM. | SANGER | |||
| SK-MEL-24 | Growth inhibition assay | Inhibition of human SK-MEL-24 cell growth in a cell viability assay, IC50 = 43.4294 μM. | SANGER | |||
| SNU-475 | Growth inhibition assay | Inhibition of human SNU-475 cell growth in a cell viability assay, IC50 = 44.5002 μM. | SANGER | |||
| HuO-3N1 | Growth inhibition assay | Inhibition of human HuO-3N1 cell growth in a cell viability assay, IC50 = 46.3321 μM. | SANGER | |||
| LAN-6 | Growth inhibition assay | Inhibition of human LAN-6 cell growth in a cell viability assay, IC50 = 46.6441 μM. | SANGER | |||
| NCI-H720 | Growth inhibition assay | Inhibition of human NCI-H720 cell growth in a cell viability assay, IC50 = 46.7503 μM. | SANGER | |||
| BB49-HNC | Growth inhibition assay | Inhibition of human BB49-HNC cell growth in a cell viability assay, IC50 = 47.6209 μM. | SANGER | |||
| TT | Growth inhibition assay | Inhibition of human TT cell growth in a cell viability assay, IC50 = 49.8459 μM. | SANGER | |||
| HT-29 | Cytotoxicity assay | In vitro cytotoxic activity against colon adenocarcinoma (HT-29) cells assayed by inhibition of [3H]-labeled thymidine incorporation, IC50 = 0.003 μM. | 11728191 | |||
| NCI-H460 | Cytotoxicity assay | In vitro cytotoxic activity against nonsmall cell lung carcinoma (NCI-H460) cells assayed by inhibition of [3H]-labeled thymidine incorporation, IC50 = 0.0078 μM. | 11728191 | |||
| MES-SA | Cytotoxicity assay | In vitro cytotoxic activity against uterine sarcoma (MES-SA) cells assayed by inhibition of [3H]-labeled thymidine incorporation, IC50 = 0.0092 μM. | 11728191 | |||
| DU-145 | Cytotoxicity assay | In vitro cytotoxic activity against prostate carcinoma (DU-145) cells assayed by inhibition of [3H]-labeled thymidine incorporation, IC50 = 0.0356 μM. | 11728191 | |||
| HepG2 | Function assay | Activity of human dCK expressed in HepG2 cells assessed as phosphorylation by coupled enzyme assay, Km = 1.4 μM. | 17101674 | |||
| SW1573 | Cytotoxicity assay | 72 hrs | Cytotoxicity against SW1573 cells after 72 hrs by SRB assay, IC50 = 8.3 μM. | 17419604 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against A549 cells after 72 hrs by SRB assay, IC50 = 13.1 μM. | 17419604 | ||
| A2780 | Growth inhibition assay | Growth inhibition of A2780 cells by SRB assay, IC50 = 0.0015 μM. | 17602464 | |||
| H460 | Growth inhibition assay | Growth inhibition of H460 cells by SRB assay, IC50 = 0.01 μM. | 17602464 | |||
| H292 | Growth inhibition assay | Growth inhibition of H292 cells by SRB assay, IC50 = 0.013 μM. | 17602464 | |||
| A549 | Growth inhibition assay | Growth inhibition of A549 cells by SRB assay, IC50 = 0.014 μM. | 17602464 | |||
| A2780 | Growth inhibition assay | Growth inhibition of A2780 cells by SRB assay in presence of dipyridamole, IC50 = 0.015 μM. | 17602464 | |||
| SW1573 | Growth inhibition assay | Growth inhibition of SW1573 cells by SRB assay, IC50 = 0.016 μM. | 17602464 | |||
| H460 | Growth inhibition assay | Growth inhibition of H460 cells by SRB assay in presence of dipyridamole, IC50 = 0.103 μM. | 17602464 | |||
| CEM | Growth inhibition assay | Growth inhibition of CEM cells by SRB assay, IC50 = 0.13 μM. | 17602464 | |||
| H292 | Growth inhibition assay | Growth inhibition of H292 cells by SRB assay in presence of dipyridamole, IC50 = 0.21 μM. | 17602464 | |||
| A549 | Growth inhibition assay | Growth inhibition of A549 cells by SRB assay in presence of dipyridamole, IC50 = 0.225 μM. | 17602464 | |||
| SW1573 | Growth inhibition assay | Growth inhibition of SW1573 cells by SRB assay in presence of dipyridamole, IC50 = 0.275 μM. | 17602464 | |||
| CEM | Growth inhibition assay | Growth inhibition of CEM cells by SRB assay in presence of dipyridamole, IC50 = 1 μM. | 17602464 | |||
| AG6000 | Growth inhibition assay | Growth inhibition of AG6000 cells by SRB assay, IC50 = 20 μM. | 17602464 | |||
| AG6000 | Growth inhibition assay | Growth inhibition of AG6000 cells by SRB assay in presence of dipyridamole, IC50 = 20 μM. | 17602464 | |||
| DU145 | Antiproliferative assay | Antiproliferative activity against human DU145 cells by MTT assay, IC50 = 0.0035 μM. | 17887663 | |||
| MDA-MB-231 | Antiproliferative assay | Antiproliferative activity against human MDA-MB-231 cells by MTT assay, IC50 = 0.0114 μM. | 17887663 | |||
| HCT15 | Cytotoxicity assay | Cytotoxicity against multidrug-resistant human HCT15 cells by thymidine incorporation assay, IC50 = 11.8 μM. | 18186604 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against multidrug-resistant human HCT116 cells by thymidine incorporation assay, IC50 = 14.3 μM. | 18186604 | |||
| OVCAR8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human OVCAR8 cells after 72 hrs by proliferative assay, IC50 = 0.0026 μM. | 18469809 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 0.0026 μM. | 18469809 | ||
| A2780/E6 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human A2780/E6 cells after 72 hrs by proliferative assay, IC50 = 0.0026 μM. | 18469809 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 0.0097 μM. | 18469809 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 0.0099 μM. | 18469809 | ||
| SF268 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human SF268 cells after 72 hrs, IC50 = 0.0103 μM. | 18469809 | ||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human SW480 cells after 72 hrs by proliferative assay, IC50 = 0.0136 μM. | 18469809 | ||
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 0.0166 μM. | 18469809 | ||
| SF539 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SF539 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 0.0198 μM. | 18469809 | ||
| Jurkat | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human Jurkat cells after 72 hrs by proliferative assay, IC50 = 0.0453 μM. | 18469809 | ||
| COLO205 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human COLO205 cells after 72 hrs, IC50 = 0.0514 μM. | 18469809 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 0.0803 μM. | 18469809 | ||
| U2OS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U2OS cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 0.0907 μM. | 18469809 | ||
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human K562 cells after 72 hrs, IC50 = 0.7459 μM. | 18469809 | ||
| HCT116/E6 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human HCT116/E6 cells after 72 hrs by proliferative assay, IC50 = 0.8965 μM. | 18469809 | ||
| MCF7 | Antitumor assay | 48 hrs | Antitumor activity against human MCF7 cells after 48 hrs by MTS assay, GI50 = 0.01 μM. | 18588281 | ||
| HT29 | Antitumor assay | 48 hrs | Antitumor activity against human HT29 cells after 48 hrs by MTS assay, GI50 = 0.03 μM. | 18588281 | ||
| CaCo2 | Antitumor assay | 48 hrs | Antitumor activity against human CaCo2 cells after 48 hrs by MTS assay, GI50 = 0.18 μM. | 18588281 | ||
| K562 | Antitumor assay | 48 hrs | Antitumor activity against human K562 cells after 48 hrs by MTS assay, GI50 = 0.32 μM. | 18588281 | ||
| A2780 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A2780 cells after 72 hrs by MTT assay, IC50 = 0.31 μM. | 19362474 | ||
| Bel7402 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 0.84 μM. | 19362474 | ||
| SW1990 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW1990 cells after 72 hrs by MTT assay, IC50 = 1.2 μM. | 19362474 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 1.4 μM. | 19362474 | ||
| Capan2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Capan2 cells after 72 hrs by MTT assay, IC50 = 1.7 μM. | 19362474 | ||
| HCT8 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT8 cells after 72 hrs by MTT assay, IC50 = 1.74 μM. | 19362474 | ||
| BxPC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BxPC3 cells after 72 hrs by MTT assay, IC50 = 2.9 μM. | 19362474 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 3.28 μM. | 19362474 | ||
| PANC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PANC1 cells after 72 hrs by MTT assay, IC50 = 5.6 μM. | 19362474 | ||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells by sulforhodamine B method, IC50 = 0.005 μM. | 19691349 | |||
| PC3 | Cytotoxicity assay | Cytotoxicity against human PC3 cells by sulforhodamine B method, IC50 = 0.04 μM. | 19691349 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by sulforhodamine B method, IC50 = 0.09 μM. | 19691349 | |||
| NCI-H23 | Cytotoxicity assay | 5 days | Cytotoxicity against human NCI-H23 cells after 5 days by SRB assay, GI50 = 0.002 μM. | 19929004 | ||
| HCT15 | Cytotoxicity assay | 5 days | Cytotoxicity against human HCT15 cells after 5 days by SRB assay, GI50 = 0.003 μM. | 19929004 | ||
| BT549 | Cytotoxicity assay | 5 days | Cytotoxicity against human BT549 cells after 5 days by SRB assay, GI50 = 0.004 μM. | 19929004 | ||
| PC3 | Cytotoxicity assay | 5 days | Cytotoxicity against human PC3 cells after 5 days by SRB assay, GI50 = 0.006 μM. | 19929004 | ||
| HuH7 | Cytotoxicity assay | Cytotoxicity against human HuH7 cells by MTS assay, CC50 = 0.1 μM. | 20580554 | |||
| HB-1 | Antiviral assay | 24 hrs | Antiviral activity against HCV 1a infected in human HB-1 cells assessed as inhibition of viral RNA replication after 24 hrs, EC50 = 1 μM. | 20580554 | ||
| OVCAR8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient OVCAR8 cells after 72 hrs by celltiter-glo assay, IC50 = 0.003 μM. | 20873740 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient PC3 cells after 72 hrs by celltiter-glo assay, IC50 = 0.003 μM. | 20873740 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 0.006 μM. | 20873740 | ||
| SF268 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient SF268 cells after 72 hrs by celltiter-glo assay, IC50 = 0.01 μM. | 20873740 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 0.01 μM. | 20873740 | ||
| SF539 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SF539 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 0.02 μM. | 20873740 | ||
| Jurkat | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient Jurkat cells after 72 hrs by celltiter-glo assay, IC50 = 0.03 μM. | 20873740 | ||
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 0.035 μM. | 20873740 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 0.08 μM. | 20873740 | ||
| U2OS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U2OS cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 0.18 μM. | 20873740 | ||
| HCT116-E6 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116-E6 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 0.5 μM. | 20873740 | ||
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient K562 cells after 72 hrs by celltiter-glo assay, IC50 = 0.6 μM. | 20873740 | ||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient SW480 cells after 72 hrs by celltiter-glo assay, IC50 = 1.7 μM. | 20873740 | ||
| COLO205 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient COLO205 cells after 72 hrs by celltiter-glo assay, IC50 = 3 μM. | 20873740 | ||
| Colo-357 | Cytotoxicity assay | Cytotoxicity against human Colo-357 cells by crystal violet staining, IC50 = 0.00036 μM. | 20930123 | |||
| PANC1 | Cytotoxicity assay | Cytotoxicity against human PANC1 cells by crystal violet staining, IC50 = 0.001 μM. | 20930123 | |||
| T3M4 | Cytotoxicity assay | Cytotoxicity against human T3M4 cells by crystal violet staining, IC50 = 0.0015 μM. | 20930123 | |||
| Patu-T | Cytotoxicity assay | Cytotoxicity against human Patu-T cells by crystal violet staining, IC50 = 0.0028 μM. | 20930123 | |||
| Patu-S | Cytotoxicity assay | Cytotoxicity against human Patu-S cells by crystal violet staining, IC50 = 0.0032 μM. | 20930123 | |||
| Patu-02 | Cytotoxicity assay | Cytotoxicity against human Patu-02 cells by crystal violet staining, IC50 = 0.0034 μM. | 20930123 | |||
| DAN-G | Cytotoxicity assay | Cytotoxicity against human DAN-G cells by crystal violet staining, IC50 = 0.0034 μM. | 20930123 | |||
| Aspc-1 | Cytotoxicity assay | Cytotoxicity against human Aspc-1 cells by crystal violet staining, IC50 = 0.004 μM. | 20930123 | |||
| BV173 | Cytotoxicity assay | 3 days | Cytotoxicity against human BV173 cells after 3 days by MTT assay, IC50 = 0.001 μM. | 21711054 | ||
| MES-SA | Cytotoxicity assay | 3 days | Cytotoxicity against human MES-SA cells after 3 days by MTT assay, IC50 = 0.005 μM. | 21711054 | ||
| K562 | Cytotoxicity assay | 3 days | Cytotoxicity against human paclitaxel resistant K562 cells after 3 days by MTT assay, IC50 = 0.006 μM. | 21711054 | ||
| CT26 | Cytotoxicity assay | 3 days | Cytotoxicity against mouse CT26 cells after 3 days by MTT assay, IC50 = 0.006 μM. | 21711054 | ||
| EL4 | Cytotoxicity assay | 3 days | Cytotoxicity against mouse EL4 cells after 3 days by MTT assay, IC50 = 0.007 μM. | 21711054 | ||
| L1210 | Cytotoxicity assay | 3 days | Cytotoxicity against mouse L1210 cells after 3 days by MTT assay, IC50 = 0.007 μM. | 21711054 | ||
| BT549 | Cytotoxicity assay | 3 days | Cytotoxicity against human BT549 cells after 3 days by MTT assay, IC50 = 0.008 μM. | 21711054 | ||
| P388D1 | Cytotoxicity assay | 3 days | Cytotoxicity against mouse P388D1 cells after 3 days by MTT assay, IC50 = 0.019 μM. | 21711054 | ||
| CEM-DNR-bulk | Cytotoxicity assay | 3 days | Cytotoxicity against human CEM-DNR-bulk cells after 3 days by MTT assay, IC50 = 0.022 μM. | 21711054 | ||
| HPAC | Cytotoxicity assay | 3 days | Cytotoxicity against human HPAC cells after 3 days by MTT assay, IC50 = 0.073 μM. | 21711054 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells after 3 days by MTT assay, IC50 = 0.149 μM. | 21711054 | ||
| MDA-MB-231 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-231 cells after 3 days by MTT assay, IC50 = 0.245 μM. | 21711054 | ||
| C6 | Cytotoxicity assay | 3 days | Cytotoxicity against rat C6 cells after 3 days by MTT assay, IC50 = 0.504 μM. | 21711054 | ||
| LNCAP | Cytotoxicity assay | 3 days | Cytotoxicity against human LNCAP cells after 3 days by MTT assay, IC50 = 0.512 μM. | 21711054 | ||
| K562 | Cytotoxicity assay | 3 days | Cytotoxicity against human K562 cells after 3 days by MTT assay, IC50 = 0.718 μM. | 21711054 | ||
| SK-N-AS | Cytotoxicity assay | 3 days | Cytotoxicity against human SK-N-AS cells after 3 days by MTT assay, IC50 = 1.1 μM. | 21711054 | ||
| U87MG | Cytotoxicity assay | 3 days | Cytotoxicity against human U87MG cells after 3 days by MTT assay, IC50 = 1.49 μM. | 21711054 | ||
| HT-29 | Cytotoxicity assay | 3 days | Cytotoxicity against human HT-29 cells after 3 days by MTT assay, IC50 = 1.53 μM. | 21711054 | ||
| NCI-H146 | Cytotoxicity assay | 3 days | Cytotoxicity against human NCI-H146 cells after 3 days by MTT assay, IC50 = 2.78 μM. | 21711054 | ||
| HeLa | Cytotoxicity assay | 3 days | Cytotoxicity against human HeLa cells after 3 days by MTT assay, IC50 = 4.12 μM. | 21711054 | ||
| SK-MEL-2 | Cytotoxicity assay | 3 days | Cytotoxicity against human SK-MEL-2 cells after 3 days by MTT assay, IC50 = 7.11 μM. | 21711054 | ||
| BJ | Cytotoxicity assay | 3 days | Cytotoxicity against human BJ cells after 3 days by MTT assay, IC50 = 9.88 μM. | 21711054 | ||
| PANC1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PANC1 cells after 48 hrs by WST-8 assay, IC50 = 0.11 μM. | 22342146 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by WST-8 assay, IC50 = 0.23 μM. | 22342146 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by WST-8 assay, IC50 = 0.32 μM. | 22342146 | ||
| ACHN | Cytotoxicity assay | 48 hrs | Cytotoxicity against human ACHN cells after 48 hrs by WST-8 assay, IC50 = 0.48 μM. | 22342146 | ||
| Calu1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Calu1 cells after 48 hrs by WST-8 assay, IC50 = 0.52 μM. | 22342146 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, GI50 = 3.64 μM. | 22512908 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, LC50 = 8.71 μM. | 22512908 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, TGI = 9.05 μM. | 22512908 | ||
| MIAPaCa2 | Growth inhibition assay | 48 hrs | Growth inhibition of human MIAPaCa2 cells after 48 hrs by SRB assay, GI50 = 35.83 μM. | 22512908 | ||
| DMS53 | Antiproliferative assay | 72 hrs | Antiproliferative activity at human DMS53 cells after 72 hrs by MTT assay, IC50 = 0.009 μM. | 22861499 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity at human A549 cells after 72 hrs by MTT assay, IC50 = 0.02 μM. | 22861499 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by resazurin assay, IC50 = 0.05 μM. | 22944119 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by resazurin assay, IC50 = 0.06 μM. | 22944119 | ||
| MIAPaCa2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MIAPaCa2 cells after 72 hrs by resazurin assay, IC50 = 0.06 μM. | 22944119 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, GI50 = 0.00364 μM. | 23094992 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, LC50 = 0.00871 μM. | 23094992 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, TGI = 0.00905 μM. | 23094992 | ||
| MIAPaCa2 | Growth inhibition assay | 48 hrs | Growth inhibition of human MIAPaCa2 cells after 48 hrs by SRB assay, GI50 = 0.03583 μM. | 23094992 | ||
| MIAPaCa2 | Growth inhibition assay | 48 hrs | Growth inhibition of human MIAPaCa2 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| MIAPaCa2 | Growth inhibition assay | 48 hrs | Growth inhibition of human MIAPaCa2 cells after 48 hrs by SRB assay, LC50 = 0.1 μM. | 23094992 | ||
| BL21(DE3) | Function assay | 1 min | Drug metabolism assessed as 2',2'-difluorodeoxycytidine formation incubated for 1 min in presence of human recombinant N-terminal His-tagged DCK Pro122Ser mutant expressed in Escherichia coli BL21(DE3) cells by liquid chromatography-tandem mass spectromet, Km = 1.43 μM. | 23230131 | ||
| BL21(DE3) | Function assay | 1 min | Drug metabolism assessed as 2',2'-difluorodeoxycytidine formation incubated for 1 min in presence of human recombinant N-terminal His-tagged DCK Ile24Val mutant expressed in Escherichia coli BL21(DE3) cells by liquid chromatography-tandem mass spectrometr, Km = 1.52 μM. | 23230131 | ||
| BL21(DE3) | Function assay | 1 min | Drug metabolism assessed as 2',2'-difluorodeoxycytidine formation incubated for 1 min in presence of human recombinant N-terminal His-tagged DCK Ala119Gly mutant expressed in Escherichia coli BL21(DE3) cells by liquid chromatography-tandem mass spectromet, Km = 1.75 μM. | 23230131 | ||
| BL21(DE3) | Function assay | 1 min | Activity of human recombinant N-terminal His-tagged wild type DCK expressed in Escherichia coli BL21(DE3) cells assessed as enzyme-mediated 2',2'-difluorodeoxycytidine formation incubated for 1 min by liquid chromatography-tandem mass spectrometry, Km = 2.15 μM. | 23230131 | ||
| MiaPaCa | Cytotoxicity assay | 6 days | Cytotoxicity against human MiaPaCa cells assessed as inhibition of cell viability after 6 days by MTT assay, IC50 = 3 μM. | 23360104 | ||
| MiaPaCa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MiaPaCa cells after 72 hrs by MTT assay, IC50 = 17.1 μM. | 23489626 | ||
| CV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against african green monkey CV1 cells after 72 hrs by MTT assay, IC50 = 21.9 μM. | 23489626 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells after 48 hrs by MTT assay, IC50 = 1.95 μM. | 23968824 | ||
| COLO205 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human COLO205 cells after 48 hrs by MTT assay, IC50 = 3.24 μM. | 23968824 | ||
| COLO320DM | Cytotoxicity assay | 48 hrs | Cytotoxicity against human COLO320DM cells after 48 hrs by MTT assay, IC50 = 3.92 μM. | 23968824 | ||
| U373-MAGI | Antiviral assay | 2 hrs | Antiviral activity against HIV1 infected in human U373-MAGI cells incubated for 2 hrs prior to viral infection followed by compound washout after 24 hrs measured 72 hrs post-infection by flow cytometry, EC50 = 0.0275 μM. | 24120088 | ||
| SW1990 | Cytotoxicity assay | Cytotoxic activity against human SW1990 cells, IC50 = 1.6 μM. | 24195466 | |||
| MCF7 | Cytotoxicity assay | 2 days | Cytostatic activity against human MCF7 cells after 2 days by coulter counter analysis, IC50 = 0.0072 μM. | 24341356 | ||
| HeLa | Cytotoxicity assay | 4 days | Cytostatic activity against human HeLa cells after 4 days by coulter counter analysis, IC50 = 0.0099 μM. | 24341356 | ||
| L1210 | Cytotoxicity assay | 2 days | Cytostatic activity against mouse L1210 cells after 2 days by coulter counter analysis, IC50 = 0.013 μM. | 24341356 | ||
| CEM | Cytotoxicity assay | 3 days | Cytostatic activity against human CEM cells after 3 days by coulter counter analysis, IC50 = 0.069 μM. | 24341356 | ||
| CEM | Cytotoxicity assay | 3 days | Cytostatic activity against human dCK-deficient CEM cells after 3 days by coulter counter analysis, IC50 = 7.6 μM. | 24341356 | ||
| FTC-133 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human FTC-133 cells after 24 hrs by MTT assay, IC50 = 3.36 μM. | 24436994 | ||
| 8305C | Cytotoxicity assay | 24 hrs | Cytotoxicity against human 8305C cells after 24 hrs by MTT assay, IC50 = 4.53 μM. | 24436994 | ||
| RT112 | Cytotoxicity assay | Cytotoxicity against human RT112 cells assessed as cell viability by MTT assay in absence of dCK substrate deoxycytidine, EC50 = 0.0014 μM. | 24471998 | |||
| L1210 | Cytotoxicity assay | 48 hrs | Cytostatic activity against mouse L1210 cells after 48 hrs by coulter counter analysis, IC50 = 0.013 μM. | 24471998 | ||
| CEM | Cytotoxicity assay | 72 hrs | Cytostatic activity against human CEM cells after 72 hrs by coulter counter analysis, IC50 = 0.086 μM. | 24471998 | ||
| RT112 | Cytotoxicity assay | Cytotoxicity against human RT112 cells assessed as cell viability by MTT assay in presence of dCK substrate deoxycytidine, EC50 = 0.1049 μM. | 24471998 | |||
| BxPC3 | Cytotoxicity assay | 72 hrs | Cytostatic activity against human BxPC3 cells after 72 hrs by MTS assay, IC50 = 0.67 μM. | 24471998 | ||
| MIAPaCa2 | Cytotoxicity assay | 72 hrs | Cytostatic activity against human MIAPaCa2 cells after 72 hrs by MTS assay, IC50 = 1.04 μM. | 24471998 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells assessed as reduction of cell survival after 48 hrs by MTT assay, IC50 = 0.05 μM. | 24631359 | ||
| CFPAC-1 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human CFPAC-1 cells assessed as reduction of cell survival after 96 hrs by MTT assay, IC50 = 0.35 μM. | 24631359 | ||
| CFPAC-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CFPAC-1 cells assessed as reduction of cell survival after 72 hrs by MTT assay, IC50 = 0.47 μM. | 24631359 | ||
| HeLa | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HeLa cells assessed as reduction of cell survival after 96 hrs by MTT assay, IC50 = 0.9 μM. | 24631359 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells assessed as reduction of cell survival after 72 hrs by MTT assay, IC50 = 10 μM. | 24631359 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells assessed as cell viability after 24 hrs by WST-1 assay, LD50 = 0.004 μM. | 24786915 | ||
| HCT116 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HCT116 cells assessed as cell viability after 24 hrs by WST-1 assay, LD50 = 0.005 μM. | 24786915 | ||
| RAW264.7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against mouse RAW264.7 cells assessed as cell viability after 24 hrs by WST-1 assay, LD50 = 0.005 μM. | 24786915 | ||
| MOLT4 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MOLT4 cells assessed as cell viability after 24 hrs by WST-1 assay, LD50 = 0.008 μM. | 24786915 | ||
| Jurkat | Cytotoxicity assay | 24 hrs | Cytotoxicity against human Jurkat cells assessed as cell viability after 24 hrs by WST-1 assay, LD50 = 0.012 μM. | 24786915 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells assessed as cell viability after 24 hrs by WST-1 assay, LD50 = 0.023 μM. | 24786915 | ||
| BxPC3 | Cytotoxicity assay | 5 days | Cytotoxicity against human BxPC3 cells after 5 days by PrestoBlue assay, EC50 = 0.01 μM. | 24867590 | ||
| MIAPaCa2 | Cytotoxicity assay | 5 days | Cytotoxicity against human MIAPaCa2 cells after 5 days by PrestoBlue assay, EC50 = 2 μM. | 24867590 | ||
| SW1990 | Cytotoxicity assay | Cytotoxicity against human SW1990 cells assessed as reduction in cell viability, IC50 = 2.2 μM. | 25105722 | |||
| MDA-MB-231 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 0.025 μM. | 25350923 | ||
| MDA-MB-231 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MDA-MB-231 cells after 24 hrs by MTT colorimetric assay, IC50 = 0.19 μM. | 25703296 | ||
| MIAPaCa2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MIAPaCa2 cells after 24 hrs by MTT colorimetric assay, IC50 = 0.6 μM. | 25703296 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTT colorimetric assay, IC50 = 3.3 μM. | 25703296 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells treated at LA + compound sequent. compound for 24 hrs, LA + compound for 24 hrs additional, total 48 hrs 2:1 and 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0039 μM. | 25874330 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells treated at 2:1 and 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0039 μM. | 25874330 | ||
| MRC5 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MRC5 cells treated at 2:1 and 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0039 μM. | 25874330 | ||
| T24 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human T24 cells treated at 2:1 and 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0039 μM. | 25874330 | ||
| T24 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human T24 cells treated at LA + compound sequent. compound for 24 hrs, LA + compound for 24 hrs additional, total 48 hrs 2:1 and 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0039 μM. | 25874330 | ||
| MRC5 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MRC5 cells treated at LA + compound sequent. compound for 24 hrs, LA + compound for 24 hrs additional, total 48 hrs 2:1 and 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0039 μM. | 25874330 | ||
| MRC5 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MRC5 cells after 48 hrs by SRB assay, IC50 = 0.0063 μM. | 25874330 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by SRB assay, IC50 = 0.0068 μM. | 25874330 | ||
| MRC5 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MRC5 cells treated at LA + compound sequent. LA for 24 hrs), LA + GEM (24 hrs additional, total 48 hrs) 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0068 μM. | 25874330 | ||
| T24 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human T24 cells after 48 hrs by SRB assay, IC50 = 0.0069 μM. | 25874330 | ||
| T24 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human T24 cells treated at LA + compound sequent. LA for 24 hrs), LA + GEM (24 hrs additional, total 48 hrs) 5:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.017 μM. | 25874330 | ||
| T24 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human T24 cells treated at LA + compound sequent. LA for 24 hrs), LA + GEM (24 hrs additional, total 48 hrs) 2:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.018 μM. | 25874330 | ||
| MRC5 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MRC5 cells treated at LA + compound sequent. LA for 24 hrs), LA + GEM (24 hrs additional, total 48 hrs) 2:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.0216 μM. | 25874330 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells treated at LA + compound sequent. LA for 24 hrs), LA + GEM (24 hrs additional, total 48 hrs) 2:1 alpha-lipoic acid to compound ratio after 48 hrs by SRB assay in presence of alpha-lipoic acid, IC50 = 0.029 μM. | 25874330 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 0.15 μM. | 26025875 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by MTT assay, IC50 = 0.52 μM. | 26025875 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50 = 0.65 μM. | 26025875 | ||
| Ramos | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Ramos cells assessed as reduction in cell viability after 72 hrs by MTS assay, EC50 = 0.00003 μM. | 27032331 | ||
| Jurkat | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Jurkat cells assessed as reduction in cell viability after 72 hrs by MTS assay, EC50 = 0.023 μM. | 27032331 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells assessed as reduction in cell viability after 72 hrs by MTS assay, EC50 = 0.065 μM. | 27032331 | ||
| B16-F10-Luc-G5 | Antiproliferative assay | 24 hrs | Antiproliferative activity against mouse B16-F10-Luc-G5 cells assessed as cell growth inhibition measured after 24 hrs by luciferase gene assay, IC50 = 11 μM. | 27349332 | ||
| SW1990 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SW1990 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 2.3 μM. | 27966950 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells incubated for 72 hrs under normoxic condition by MTT assay, IC50 = 0.57 μM. | 28075592 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells incubated for 72 hrs under hypoxic condition by MTT assay, IC50 = 8.9 μM. | 28075592 | ||
| CCRF-CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against daunorubicin resistant human CCRF-CEM cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.02 μM. | 28221790 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.03 μM. | 28221790 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.05 μM. | 28221790 | ||
| K562-TAX | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562-TAX cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.05 μM. | 28221790 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.1 μM. | 28221790 | ||
| CEM-DNR-bulk | Cytotoxicity assay | 72 hrs | Cytotoxicity against mrp-1/mdr-1 overexpressing human CEM-DNR-bulk cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.1 μM. | 28221790 | ||
| U2OS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U2OS cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.18 μM. | 28221790 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against p53 deficient human HCT116 cells assessed as cell growth inhibition after 72 hrs by MTS assay, IC50 = 0.41 μM. | 28221790 | ||
| PANC1 | Growth inhibition assay | 72 hrs | Growth inhibition of human PANC1 cells after 72 hrs by WST8 assay, GI50 = 5.8 μM. | 28495081 | ||
| BxPC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human BxPC3 cells after 72 hrs by CCK8 assay, IC50 = 20.2 μM. | 28576633 | ||
| AsPC1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human AsPC1 cells after 72 hrs by CCK8 assay, IC50 = 26.8 μM. | 28576633 | ||
| PANC1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PANC1 cells after 72 hrs by CCK8 assay, IC50 = 30.4 μM. | 28576633 | ||
| MDA-MB-231 | Growth inhibition assay | 72 hrs | Growth inhibition of human MDA-MB-231 cells after 72 hrs by Hoechst 33258 dye based fluorescence assay, IC50 = 0.03 μM. | 29253340 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells after 72 hrs by Hoechst 33258 dye based fluorescence assay, IC50 = 0.03 μM. | 29253340 | ||
| MDA-MB-231 | Antiproliferative assay | Antiproliferative activity against human MDA-MB-231 cells, GI50 = 0.000251 μM. | 29301085 | |||
| SW620 | Antiproliferative assay | Antiproliferative activity against human SW620 cells, GI50 = 0.00084 μM. | 29301085 | |||
| H460 | Antiproliferative assay | Antiproliferative activity against human H460 cells harboring dominant negative p53 construct, GI50 = 0.002085 μM. | 29301085 | |||
| MIAPaCa2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MIAPaCa2 cells assessed as decrease in cell proliferation after 72 hrs by MTT assay, IC50 = 0.11 μM. | 29328656 | ||
| MIAPaCa2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against gemcitabine-resistant human MIAPaCa2 cells assessed as decrease in cell proliferation after 72 hrs by MTT assay, IC50 = 3.3 μM. | 29328656 | ||
| BxPC3 | Growth inhibition assay | 96 hrs | Growth inhibition of human BxPC3 cells after 96 hrs by SRB assay, IC50 = 0.006 μM. | 29356532 | ||
| Capan1 | Growth inhibition assay | 96 hrs | Growth inhibition of human Capan1 cells after 96 hrs by SRB assay, IC50 = 0.019 μM. | 29356532 | ||
| MIAPaCa2 | Antiproliferative assay | 70 hrs | Antiproliferative activity against human MIAPaCa2 cells after 70 hrs by alamar blue assay, IC50 = 0.12 μM. | 29471119 | ||
| PANC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PANC1 cells after 72 hrs under normoxic condition by MTT assay, IC50 = 0.4 μM. | 29656202 | ||
| PANC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PANC1 cells after 72 hrs under hypoxic condition by MTT assay, IC50 = 1.7 μM. | 29656202 | ||
| MDA-MB-231 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 0.0364 μM. | 29795767 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 53 mg/mL
(201.36 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 263.2 | Formula | C9H11F2N3O4 |
Storage (From the date of receipt) | powder, 4°C (in the dark) ; in solvent,-80°C (in the dark) |
|---|---|---|---|---|---|
| CAS No. | 95058-81-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LY-188011, NSC 613327 | Smiles | C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)(F)F | ||
Mechanism of Action
| In vitro |
Gemcitabine results in 50% inhibition of growth in the CCRF-CEM human leukemia cell culture assay with IC50 of 1 ng/ml. This compound combined with deoxycytidine provides about a 1000-fold decrease in biological activity. This chemical combined with C225 results in additive cytotoxic effects that increased with increasing gemcitabine concentrations in human pancreatic carcinoma L3.6pl cells. It combined with Cisplatin results in synergistic effect in wild-type A2780 and cisplatin-resistant ADDP cells. |
|---|---|
| In vivo |
Gemcitabine combined with C225 results in growth inhibition, tumor regression, and abrogation of metastasis in L3.6pl tumors established in the pancreas of nude mice. This compound treatment alone reduces median tumor volume from 538 to 152 mm3. It reduces the production of vascular endothelial growth factor and interleukin 8 in gemcitabine-treated tumors. This chemical is able to dramatically and specifically reduces the number of myeloid suppressor cells found in the spleens of animals bearing large tumors with no significant reductions in CD4(+) T cells, CD8(+) T cells, NK cells, macrophages, or B cells. It combined with curcumin shows significant reductions in volume (P = 0.008 versus control; P = 0.036 versus gemcitabine alone), Ki-67 proliferation index (P = 0.030 versus control), NF-kappaB activation, and expression of NF-kappaB-regulated gene products (cyclin D1, c-myc, Bcl-2, Bcl-xL, cellular inhibitor of apoptosis protein-1, cyclooxygenase-2, matrix metalloproteinase, and vascular endothelial growth factor) compared with tumors from control mice treated with olive oil only. This compound combined with Curcumin is also highly effective in suppressing angiogenesis as indicated by a decrease in CD31(+) microvessel density. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p70S6K1 / p-S6 / HIF-1α PARP / Cleaved PARP / Cleaved caspase-3 / phopho-p38 / p38 / p-JNK / JNK / p-c-Jun |
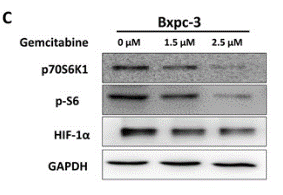
|
27765914 |
| Immunofluorescence | Bim1 |
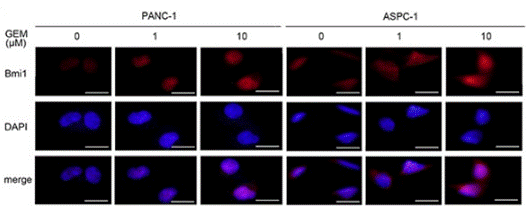
|
27177084 |
| Growth inhibition assay | Cell viability |
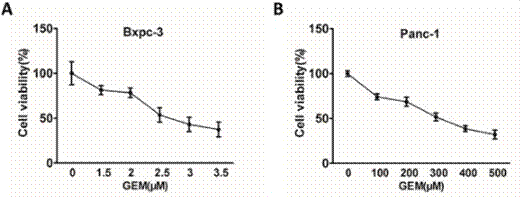
|
27765914 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06320301 | Recruiting | Biliary Tract Cancer|Gemox Chemotherapy |
Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University|Suzhou Suncadia Biopharmaceuticals Co. Ltd. |
April 1 2024 | Phase 2 |
| NCT06046794 | Not yet recruiting | Cancer Of Pancreas |
Institut Paoli-Calmettes |
February 1 2024 | Not Applicable |
| NCT06199466 | Recruiting | Metastatic Pancreatic Cancer |
M.D. Anderson Cancer Center|280Bio Inc |
January 22 2024 | Phase 1 |
| NCT06055348 | Not yet recruiting | Serous Ovarian Cancer|Advanced Ovarian Cancer |
Biocity Biopharmaceutics Co. Ltd. |
October 30 2023 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
What is the difference between it (S1714) and Gemcitabine HCl (S1149)?
Answer:
Its HCl salt form has the same biological activities. The free base (S1714) dissolves better in DMSO, and the hydrochloride (S1149) dissolves better in water.






































