
- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Camptothecin (CPT)
ADC Cytotoxin inhibitor
research use only
Camptothecin (CPT) is a specific inhibitor of DNA topoisomerase I (Topo I) with IC50 of 0.68 μM in a cell-free assay. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Phase 2.
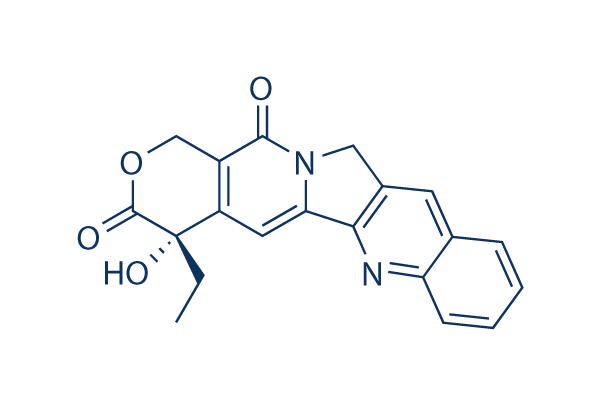
Camptothecin (CPT) Chemical Structure
Molecular Weight: 348.35
Purity & Quality Control
Batch:
Purity:
99.79%
99.79
Camptothecin (CPT) Related Products
| Related Products | SN-38 Triptolide (+)-Bicuculline Rutin Artemisinin Pinocembrin BHQ Harmine hydrochloride Psoralen Lappaconite HBr Luteoloside Lycorine hydrochloride Tetrahydropalmatine hydrochloride | Click to Expand |
|---|---|---|
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Apoptosis Compound Library DNA Damage/DNA Repair compound Library Cell Cycle compound library | Click to Expand |
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| P388 | Growth inhibitory assay | IC50=32 nM | 10346933 | |||
| P388CPT5 R | Growth inhibitory assay | IC50=2.8 μM | 10346933 | |||
| KBwt | Growth inhibitory assay | IC50=40 nM | 10411476 | |||
| KBMDR | Growth inhibitory assay | IC50=70 nM | 10411476 | |||
| KBV20C | Growth inhibitory assay | IC50=30 nM | 10411476 | |||
| KB7D | Growth inhibitory assay | IC50=35 nM | 10411476 | |||
| KBCamp | Growth inhibitory assay | IC50=1.04 μM | 10411476 | |||
| HT29 | cytotoxicity assay | IC50=80 nM | 10841808 | |||
| A549 | cytotoxicity assay | IC50=67 nM | 10841808 | |||
| T24 | cytotoxicity assay | IC50=88 nM | 10841808 | |||
| HOP-62 | Growth inhibitory assay | IC50=10 nM | 11020283 | |||
| HCT-116 | Growth inhibitory assay | IC50=30 nM | 11020283 | |||
| SF-539 | Growth inhibitory assay | IC50=10 nM | 11020283 | |||
| UACC-62 | Growth inhibitory assay | IC50=10 nM | 11020283 | |||
| OVCAR-3 | Growth inhibitory assay | IC50=220 nM | 11020283 | |||
| SN12C | Growth inhibitory assay | IC50=20 nM | 11020283 | |||
| DU-145 | Growth inhibitory assay | IC50=10 nM | 11020283 | |||
| MDA-MB-435 | Growth inhibitory assay | IC50=40 nM | 11020283 | |||
| WiDr | cytotoxicity assay | DMSO | IC50=17 nM | 11334569 | ||
| A549 | cytotoxicity assay | DMSO | IC50=14 nM | 11334569 | ||
| MKN45 | cytotoxicity assay | DMSO | IC50=17 nM | 11334569 | ||
| SK-OV-3 | cytotoxicity assay | DMSO | IC50=20 nM | 11334569 | ||
| H128 | cytotoxicity assay | DMSO | IC50=18 nM | 11334569 | ||
| SK-BR-3 | cytotoxicity assay | DMSO | IC50=20 nM | 11334569 | ||
| LX-1 | cytotoxicity assay | DMSO | IC50=120 nM | 11858737 | ||
| HCT116 | cytotoxicity assay | DMSO | IC50=9 nM | 11858737 | ||
| A2780 | cytotoxicity assay | DMSO | IC50=4 nM | 11858737 | ||
| IMR-32 | cytotoxicity assay | ~10 μM | DMSO | IC50=2.21 nM | 12617894 | |
| P388 | cytotoxicity assay | IC50=13 nM | 12620081 | |||
| Lewis | cytotoxicity assay | IC50=33 nM | 12620081 | |||
| JLC | cytotoxicity assay | IC50=5.6 nM | 12620081 | |||
| HT-29 | cytotoxicity assay | IC50=1.4 μM | 12639541 | |||
| Caki-2 | cytotoxicity assay | IC50=3.96 μM | 12639541 | |||
| A549 | cytotoxicity assay | IC50=2.53 μM | 12639541 | |||
| HEC-1-B | cytotoxicity assay | IC50=8.64 μM | 12639541 | |||
| HL-60 | cytotoxicity assay | IC50=66 nM | 12639541 | |||
| Col2 | cytotoxicity assay | ~1 μM | ED50=57 nM | 15043407 | ||
| HUVEC | cytotoxicity assay | ~1 μM | ED50=258 nM | 15043407 | ||
| KB | cytotoxicity assay | ~1 μM | ED50=22 nM | 15043407 | ||
| LCNaP | cytotoxicity assay | ~1 μM | ED50=28 nM | 15043407 | ||
| Lu1 | cytotoxicity assay | ~1 μM | ED50=29 nM | 15043407 | ||
| RPMI8402 | cytotoxicity assay | ~10 μM | IC50=6 nM | 15482929 | ||
| CPT-K5 | cytotoxicity assay | ~10 μM | IC50>10 μM | 15482929 | ||
| P388 | cytotoxicity assay | ~10 μM | IC50=14 nM | 15482929 | ||
| P388/CPT45 | cytotoxicity assay | ~10 μM | IC50>10 μM | 15482929 | ||
| KB3-1 | cytotoxicity assay | ~10 μM | IC50=40 nM | 15482929 | ||
| KBV-1 + MDR1 | cytotoxicity assay | ~10 μM | IC50=440 nM | 15482929 | ||
| KBH | cytotoxicity assay | ~10 μM | IC50=440 nM | 15482929 | ||
| HOP-62 | cytotoxicity assay | ~10 μM | GI50=10 nM | 15509164 | ||
| HCT-116 | cytotoxicity assay | ~10 μM | GI50=30 nM | 15509164 | ||
| F-539 | cytotoxicity assay | ~10 μM | GI50=10 nM | 15509164 | ||
| UACC-62 | cytotoxicity assay | ~10 μM | GI50=10 nM | 15509164 | ||
| OVCAR-3 | cytotoxicity assay | ~10 μM | GI50=220 nM | 15509164 | ||
| SN12C | cytotoxicity assay | ~10 μM | GI50=20 nM | 15509164 | ||
| DU-145 | cytotoxicity assay | ~10 μM | GI50=10 nM | 15509164 | ||
| MDA-MB-435 | cytotoxicity assay | ~10 μM | GI50=40 nM | 15509164 | ||
| MT-4 | cytotoxicity assay | IC50=4 nM | 17254669 | |||
| CCRF-CEMc | cytotoxicity assay | IC50=3 nM | 17254669 | |||
| WIL-2NSd | cytotoxicity assay | IC50=5 nM | 17254669 | |||
| CCRF-SB | cytotoxicity assay | IC50=3 nM | 17254669 | |||
| CRL 7065 | cytotoxicity assay | IC50=400 nM | 17254669 | |||
| SK-MEL-28b | cytotoxicity assay | IC50=40 nM | 17254669 | |||
| MCF-7 | cytotoxicity assay | IC50=40 nM | 17254669 | |||
| SKMES-1 | cytotoxicity assay | IC50=10 nM | 17254669 | |||
| HepG2 | cytotoxicity assay | IC50=30 nM | 17254669 | |||
| DU145 | cytotoxicity assay | IC50=10 nM | 17254669 | |||
| SKOV3 | cytotoxicity assay | IC50=51 nM | 9003520 | |||
| SKVLB | cytotoxicity assay | IC50=53 nM | 9003520 | |||
| HT29 | cytotoxicity assay | IC50=87.8 nM | 9003520 | |||
| KB | cytotoxicity assay | IC50=8 nM | 9003520 | |||
| A427 | Growth inhibitory assay | ~1 μM | DMSO | IC50=24 nM | 9876111 | |
| PC-3 | Growth inhibitory assay | ~1 μM | DMSO | IC50=57 nM | 9876111 | |
| K562adr | Growth inhibitory assay | ~1 μM | DMSO | IC50=57 nM | 9876111 | |
| MCF7mdr | Growth inhibitory assay | ~1 μM | DMSO | IC50=3.1 nM | 9876111 | |
| L1210 | Function assay | Concentration that inhibits the proliferation of L1210 cells by 50%, IC50=0.014μM. | 2537428 | |||
| SK-OV-3 | Cytotoxicity assay | Cytotoxicity was determined in vitro in SK-OV-3 cells (ovarian) of human tumor cell lines by using MTT assay, IC50=0.037μM. | 7853331 | |||
| SKVLB | Cytotoxicity assay | Cytotoxicity was determined in vitro in SKVLB cells(ovarian with upregulated MDRp-glycoprotein) of human tumor cell lines by using MTT assay, IC50=0.041μM. | 7853331 | |||
| HT-29 | Cytotoxicity assay | Cytotoxicity was determined in vitro in HT-29 cells(colon) of human tumor cell lines by using MTT assay, IC50=0.046μM. | 7853331 | |||
| LOX | Cytotoxicity assay | Cytotoxicity was determined in vitro in LOX cells (melanoma) of human tumor cell lines by using MTT assay, IC50=0.048μM. | 7853331 | |||
| T47D | Cytotoxicity assay | Cytotoxicity was determined in vitro in T47D cells(breast) of human tumor cell lines by using MTT assay, IC50=0.207μM. | 7853331 | |||
| L1210 | Cytotoxicity assay | Cytotoxicity was measured on cultured murine lymphoblastic leukemia cells (L1210), IC50=0.015μM. | 8246238 | |||
| HL-60 | Function assay | Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells, IC50=0.68μM. | 8410981 | |||
| KB | Growth inhibition assay | Compound concentration required to reduce the exponential growth of KB cells by 50%, CC50=0.006μM. | 9767632 | |||
| MT-4 | Growth inhibition assay | Compound concentration required to reduce the exponential growth of MT-4 cells by 50%, CC50=0.01μM. | 9767632 | |||
| COLO 205 | Cytotoxicity assay | Cytotoxicity expressed as the concentration that inhibited incorporation of [3H]thymidine into cellular DNA of human colorectal cells (COLO 205), IC50=0.0054μM. | 10479293 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity expressed as the concentration that inhibited incorporation of [3H]thymidine into cellular DNA of human hepatocellular cells (HepG2), IC50=0.0063μM. | 10479293 | |||
| H928 | Cytotoxicity assay | Cytotoxicity expressed as the concentration that inhibited incorporation of [3H]-thymidine into cellular DNA of human lung carcinoma cells (H928), IC50=0.0078μM. | 10479293 | |||
| CaSki | Cytotoxicity assay | Cytotoxicity expressed as the concentration that inhibited incorporation of [3H]thymidine into cellular DNA of human cervical cells (CaSki), IC50=0.0085μM. | 10479293 | |||
| MDA-MB-435 | Function assay | Inhibition against MDA-MB-435 human breast cancer cells in the absence of HSA, IC50=0.015μM. | 11052802 | |||
| MDA-MB-435 | Function assay | Inhibition against MDA-MB-435 human breast cancer cells in the presence of 11 mg/mL HSA, IC50=0.375μM. | 11052802 | |||
| Jurkat | Function assay | Inhibitory activity against Jurkat human leukemia cells, IC50=0.0056μM. | 11384245 | |||
| P388 | Function assay | Inhibitory activity against P388 murine leukemia cells, IC50=0.013μM. | 11384245 | |||
| Lewis lung carcinoma cells | Function assay | Inhibitory activity against Lewis lung carcinoma cells, IC50=0.033μM. | 11384245 | |||
| A549 | Function assay | Inhibition topoisomerase 1 in human A549 cells assessed as conversion of supercoiled pBR322 DNA to relaxed form, IC50=4μM. | 11430001 | |||
| HeLa | Function assay | Inhibition topoisomerase 1 in human HeLa cells assessed as conversion of supercoiled pBR322 DNA to relaxed form, IC50=9μM. | 11430001 | |||
| NIH/3T3 | Function assay | Inhibition topoisomerase 1 in mouse NIH/3T3 cells assessed as conversion of supercoiled pBR322 DNA to relaxed form, IC50=12μM. | 11430001 | |||
| COLO201 | Function assay | Inhibition topoisomerase 1 in human COLO201 cells assessed as conversion of supercoiled pBR322 DNA to relaxed form, IC50=17μM. | 11430001 | |||
| Vero | Function assay | Inhibition topoisomerase 1 in african green monkey Vero cells assessed as conversion of supercoiled pBR322 DNA to relaxed form, IC50=27μM. | 11430001 | |||
| SW626 | Cytotoxicity assay | Cytotoxicity against human SW626 cells by MTT assay, IC50=0.0287μM. | 11858760 | |||
| Lu1 | Cytotoxicity assay | Cytotoxicity against human Lu1 cells by MTT assay, IC50=0.0287μM. | 11858760 | |||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells by MTT assay, IC50=0.0287μM. | 11858760 | |||
| HOG.R5 | Cytotoxicity assay | Cytotoxicity against human HOG.R5 cells by MTT assay, IC50=0.0287μM. | 11858760 | |||
| LNCAP | Cytotoxicity assay | Cytotoxicity against human LNCAP cells by MTT assay, IC50=0.0861μM. | 11858760 | |||
| HCT116 | Function assay | Compound was tested in vitro for cytotoxicity against HCT116, human colon cancer cells (taxol-resistant) at a drug concentration producing 50% inhibition of colony formation, ID50=0.0028μM. | 11965362 | |||
| PC-3 | Function assay | Compound was tested in vitro for cytotoxicity against PC-3, human prostate cancer cells (taxol-sensitive) at a drug concentration producing 50% inhibition of colony formation, ID50=0.0038μM. | 11965362 | |||
| MCF-7ADR | Function assay | Compound was tested in vitro for cytotoxicity against MCF-7ADR, human breast cancer cells (taxol-resistant) at a drug concentration producing 50% inhibition of colony formation, ID50=0.0039μM. | 11965362 | |||
| L1210 | Function assay | Inhibitory concentration required against L1210 leukemia cells, IC50=0.03μM. | 12182872 | |||
| L1210 | Cytotoxicity assay | Cytotoxicity against L1210 leukemia cells, Concentration=0.05μM. | 12182872 | |||
| P388 | Function assay | Inhibitory activity against P388 Leukemia cells, IC50=0.007μM. | 12565975 | |||
| CCRF-CEM | Cytotoxicity assay | Cytotoxicity in human leukemic CCRF-CEM cells, IC50=5.3μM. | 14667232 | |||
| HeLa | Function assay | Concentration required to inhibit S phase and G2/M phase in HeLa cells, Concentration=0.1μM. | 15026047 | |||
| HeLa | Growth inhibition assay | Growth inhibitory concentration of compound was determined against HeLa cells by alamar blue assay, GI50=0.6μM. | 15026047 | |||
| SW620 | Growth inhibition assay | Concentration required to inhibit growth of SW620 (human colon cancer) cells in the presence of 10 uM of Compound 2e, EC50=0.00015μM. | 15857116 | |||
| SW620 | Growth inhibition assay | Concentration required to inhibit growth of SW620 (human colon cancer) cells, EC50=0.0012μM. | 15857116 | |||
| CNSSF-539 | Growth inhibition assay | Concentration required to inhibit the growth of human CNSSF-539 cells, GI50=0.01μM. | 16033260 | |||
| LungHOP-62 | Growth inhibition assay | Concentration required to inhibit the growth of human LungHOP-62 cells, GI50=0.01μM. | 16033260 | |||
| ProstateDU-145 | Growth inhibition assay | Concentration required to inhibit the growth of human ProstateDU-145 cells, GI50=0.01μM. | 16033260 | |||
| Melanoma UACC-62 | Growth inhibition assay | Concentration required to inhibit the growth of human Melanoma UACC-62 cells, GI50=0.01μM. | 16033260 | |||
| Renal SN12C | Growth inhibition assay | Concentration required to inhibit the growth of human Renal SN12C cells, GI50=0.02μM. | 16033260 | |||
| ColonHCT-11 | Growth inhibition assay | Concentration required to inhibit the growth of human ColonHCT-11 cells, GI50=0.03μM. | 16033260 | |||
| MDA-MB-435 | Growth inhibition assay | Concentration required to inhibit the growth of human Breast MDA-MB-435 cells, GI50=0.04μM. | 16033260 | |||
| OVCAR | Growth inhibition assay | Concentration required to inhibit the growth of human OvarianOVCAR cells, GI50=0.22μM. | 16033260 | |||
| U937 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U937 cells after 72 hrs by WST-8 assay, IC50=0.015μM. | 17158054 | ||
| U937 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human U937 cells after 48 hrs by WST-8 assay, IC50=0.032μM. | 17158054 | ||
| U937 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human U937 cells after 24 hrs by WST-8 assay, IC50=0.68μM. | 17158054 | ||
| T24 | Growth inhibition assay | Growth inhibition of human T24 cells by neutral red assay, IC50=0.006μM. | 17204425 | |||
| HT29 | Growth inhibition assay | Growth inhibition of human HT29 cells by neutral red assay, IC50=0.014μM. | 17204425 | |||
| MDA-MB-231 | Growth inhibition assay | Growth inhibition of human MDA-MB-231 cells by neutral red assay, IC50=0.291μM. | 17204425 | |||
| SW620 | Antiproliferative assay | Antiproliferative activity against human SW620 cells by soft agar assay, EC50=0.0021μM. | 17287122 | |||
| LNCAP | Cytotoxicity assay | Cytotoxicity against human LNCAP cells by sulforhodamine B assay, ED50=0.002μM. | 17350834 | |||
| 1A9 | Cytotoxicity assay | Cytotoxicity against human 1A9 cells by sulforhodamine B assay, ED50=0.0032μM. | 17350834 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by sulforhodamine B assay, ED50=0.0032μM. | 17350834 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by sulforhodamine B assay, ED50=0.0069μM. | 17350834 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by sulforhodamine B assay, ED50=0.0109μM. | 17350834 | |||
| KBVIN | Cytotoxicity assay | Cytotoxicity against multi drug-resistant human KBVIN cells by sulforhodamine B assay, ED50=0.015μM. | 17350834 | |||
| PC3 | Cytotoxicity assay | Cytotoxicity against human PC3 cells by sulforhodamine B assay, ED50=0.0167μM. | 17350834 | |||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells by sulforhodamine B assay, ED50=0.0209μM. | 17350834 | |||
| HOP62 | Cytotoxicity assay | Cytotoxicity against human HOP62 cells, GI50=0.01μM. | 17402722 | |||
| SF268 | Cytotoxicity assay | Cytotoxicity against human SF268 cells, GI50=0.01μM. | 17402722 | |||
| UACC62 | Cytotoxicity assay | Cytotoxicity against human UACC62 cells, GI50=0.01μM. | 17402722 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, GI50=0.01μM. | 17402722 | |||
| SN12C | Cytotoxicity assay | Cytotoxicity against human SN12C cells, GI50=0.02μM. | 17402722 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, GI50=0.03μM. | 17402722 | |||
| MDA-MA-435 | Cytotoxicity assay | Cytotoxicity against human MDA-MA-435 cells, GI50=0.04μM. | 17402722 | |||
| OVCAR-3 | Cytotoxicity assay | Cytotoxicity against human OVCAR-3 cells, GI50=0.22μM. | 17402722 | |||
| K562 | Growth inhibition assay | 5 days | Growth inhibition of K562 cells by XTT assay after 5 days, IC50=0.04μM. | 17411092 | ||
| neural precursor cells | Function assay | Inhibition of neurosphere proliferation of mouse neural precursor cells by MTT assay | 17417631 | |||
| CEM | Growth inhibition assay | Growth inhibition of human CEM cells by Alamar blue assay, GI50=0.002μM. | 17418582 | |||
| MCF7 | Growth inhibition assay | 4 days | Growth inhibition of adriamycin-resistant MCF7 cells after 4 days, GI50=0.01μM. | 17418582 | ||
| HeLa | Growth inhibition assay | 4 days | Growth inhibition of HeLa cells after 4 days, GI50=0.01μM. | 17418582 | ||
| MCF7 | Growth inhibition assay | 4 days | Growth inhibition of MCF7 cells after 4 days, GI50=0.01μM. | 17418582 | ||
| MX2 | Growth inhibition assay | Growth inhibition of topoisomerase 2 mutated mitoxantrone-resistant MX2 cells by Alamar blue assay, GI50=0.02μM. | 17418582 | |||
| HL60 | Growth inhibition assay | Growth inhibition of human HL60 cells by Almar blue assay, GI50=0.03μM. | 17418582 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells by MTT assay, IC50=0.018μM. | 17498951 | |||
| Col2 | Cytotoxicity assay | Cytotoxicity against human Col2 cells by SRB assay, IC50=0.045μM. | 17498951 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB assay, IC50=0.069μM. | 17498951 | |||
| HT1080 | Cytotoxicity assay | Cytotoxicity against human HT1080 cells by SRB assay, IC50=0.08μM. | 17498951 | |||
| SNU638 | Cytotoxicity assay | Cytotoxicity against human SNU638 cells by SRB assay, IC50=0.098μM. | 17498951 | |||
| HT29 | Apoptosis assay | 5 uM | Induction of apoptosis in human HT29 cells at 5 uM | 17512744 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by MTT method, IC50=0.5μM. | 17547457 | |||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells, EC50=0.0036μM. | 17552563 | |||
| P388 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse P388 cells after 72 hrs, IC50=0.0321μM. | 17624792 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs, IC50=0.004μM. | 17673337 | ||
| SK-OV3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SK-OV3 cells after 48 hrs, IC50=0.014μM. | 17673337 | ||
| HCT15 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT15 cells after 48 hrs, IC50=0.083μM. | 17673337 | ||
| SK-MEL-2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SK-MEL-2 cells after 48 hrs, IC50=0.165μM. | 17673337 | ||
| HOP62 | Cytotoxicity assay | Cytotoxicity against human HOP62 cells, GI50=0.01μM. | 17676830 | |||
| SF539 | Cytotoxicity assay | Cytotoxicity against human SF539 cells, GI50=0.01μM. | 17676830 | |||
| UACC62 | Cytotoxicity assay | Cytotoxicity against human UACC62 cells, GI50=0.01μM. | 17676830 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, GI50=0.01μM. | 17676830 | |||
| SN12C | Cytotoxicity assay | Cytotoxicity against human SN12C cells, GI50=0.02μM. | 17676830 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, GI50=0.03μM. | 17676830 | |||
| MDA-MB-435 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-435 cells, GI50=0.04μM. | 17676830 | |||
| OVCAR-3 | Cytotoxicity assay | Cytotoxicity against human OVCAR-3 cells, GI50=0.22μM. | 17676830 | |||
| HOP62 | Cytotoxicity assay | Cytotoxicity against human HOP62 cells, GI50=0.01μM. | 17696418 | |||
| SF539 | Cytotoxicity assay | Cytotoxicity against human SF539 cells, GI50=0.01μM. | 17696418 | |||
| UACC62 | Cytotoxicity assay | Cytotoxicity against human UACC62 cells, GI50=0.01μM. | 17696418 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, GI50=0.01μM. | 17696418 | |||
| SN12C | Cytotoxicity assay | Cytotoxicity against human SN12C cells, GI50=0.02μM. | 17696418 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, GI50=0.03μM. | 17696418 | |||
| MDA-MB-435 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-435 cells, GI50=0.04μM. | 17696418 | |||
| OVCAR-3 | Cytotoxicity assay | Cytotoxicity against human OVCAR-3 cells, GI50=0.22μM. | 17696418 | |||
| OV3 | Cytotoxicity assay | Cytotoxicity against human OV3 cells by SRB assay, IC50=0.024μM. | 17827007 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB assay, IC50=0.067μM. | 17827007 | |||
| SK-MEL-2 | Cytotoxicity assay | Cytotoxicity against human SK-MEL-2 cells by SRB assay, IC50=0.075μM. | 17827007 | |||
| HCT15 | Cytotoxicity assay | Cytotoxicity against human HCT15 cells by SRB assay, IC50=0.08μM. | 17827007 | |||
| HL60 | Apoptosis assay | 1 uM | 6 hrs | Induction of apoptosis in human HL60 cells assessed as nucleosomal DNA fragmentation at 1 uM after 6 hrs | 17950603 | |
| HL60/MX2 | Cytotoxicity assay | Cytotoxicity against DNA topoisomerase-2 deficient human HL60/MX2 cells by MTT assay, GI50=0.0015μM. | 17962028 | |||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, GI50=0.0017μM. | 17962028 | ||
| Jurkat | Growth inhibition assay | 96 hrs | Growth inhibition of human Jurkat cells after 96 hrs, IC50=0.0014μM. | 18061452 | ||
| K562 | Cytotoxicity assay | 5 days | Cytotoxicity against human K562 cells after 5 days by XTT assay, IC50=0.04μM. | 18076140 | ||
| A2780 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A2780 cells after 96 hrs by MTT assay, IC50=0.28μM. | 18095653 | ||
| A549 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A549 cells after 96 hrs by MTT assay, IC50=3.12μM. | 18095653 | ||
| HCT8 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HCT8 cells after 96 hrs by MTT assay, IC50=3.15μM. | 18095653 | ||
| BGC823 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human BGC823 cells after 96 hrs by MTT assay, IC50=9.67μM. | 18095653 | ||
| Bel7402 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human Bel7402 cells after 96 hrs by MTT assay, IC50=12.5μM. | 18095653 | ||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells, IC50=0.013μM. | 18178085 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, IC50=1μM. | 18178085 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells, IC50=1.08μM. | 18178085 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, IC50=3.2μM. | 18178085 | |||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells, IC50=4.16μM. | 18178085 | |||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by MTT assay, GI50=0.09μM. | 18180162 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, GI50=0.18μM. | 18180162 | ||
| SKHep | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SKHep cells after 48 hrs by MTT assay, GI50=0.22μM. | 18180162 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, GI50=2.8μM. | 18180162 | ||
| SAS | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SAS cells after 48 hrs by MTT assay, GI50=6μM. | 18180162 | ||
| AGS | Antiproliferative assay | 48 hrs | Antiproliferative activity against human AGS cells after 48 hrs by MTT assay, GI50=23.76μM. | 18180162 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs, IC50=0.0062μM. | 18419110 | ||
| IGROV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human IGROV1 cells after 72 hrs, IC50=0.012μM. | 18419110 | ||
| H460 | Cytotoxicity assay | 1 hr | Cytotoxicity against human H460 cells after 1 hr, IC50=0.33μM. | 18419110 | ||
| IGROV1 | Cytotoxicity assay | 1 hr | Cytotoxicity against human IGROV1 cells after 1 hr, IC50=1.65μM. | 18419110 | ||
| HT29 | Cytotoxicity assay | 1 hr | Cytotoxicity against human HT29 cells after 1 hr, IC50=3.16μM. | 18419110 | ||
| HT29 | Cytotoxicity assay | 1 hr | Cytotoxicity against human mitoxantrone-resistant HT29 cells after 1 hr, IC50=8.81μM. | 18419110 | ||
| H460 | Antiproliferative assay | 1 hr | Antiproliferative activity against human H460 cells after 1 hr of drug exposure measured after 72 hrs, IC50=0.18μM. | 18424133 | ||
| HT29 | Antiproliferative assay | 1 hr | Antiproliferative activity against human HT29 cells after 1 hr of drug exposure measured after 72 hrs, IC50=3.16μM. | 18424133 | ||
| HT29 | Antiproliferative assay | 1 hr | Antiproliferative activity against BCRP overexpressing mitoxantrone-resistant human HT29 cells after 1 hr of drug exposure measured after 72 hrs, IC50=8.81μM. | 18424133 | ||
| P388 | Cytotoxicity assay | Cytotoxicity against human P388 cells, IC50=0.000004μM. | 18511275 | |||
| RPMI-8240 | Cytotoxicity assay | Cytotoxicity against human RPMI-8240 cells, IC50=0.000004μM. | 18511275 | |||
| KB | Cytotoxicity assay | 4 days | Cytotoxicity against human KB cells after 4 days by MTT assay, IC50=0.003μM. | 18554906 | ||
| A549 | Cytotoxicity assay | 4 days | Cytotoxicity against human A549 cells after 4 days by MTT assay, IC50=0.01μM. | 18554906 | ||
| HT29 | Cytotoxicity assay | 4 days | Cytotoxicity against human HT29 cells after 4 days by MTT assay, IC50=0.09μM. | 18554906 | ||
| HepG2 | Cytotoxicity assay | 4 days | Cytotoxicity against human HepG2 cells after 4 days by MTT assay, IC50=0.13μM. | 18554906 | ||
| BEL-7402 | Cytotoxicity assay | 4 days | Cytotoxicity against human BEL-7402 cells after 4 days by MTT assay, IC50=0.17μM. | 18554906 | ||
| MCF7 | Cytotoxicity assay | 4 days | Cytotoxicity against human MCF7 cells after 4 days by MTT assay, IC50=0.2μM. | 18554906 | ||
| A2780 | Cytotoxicity assay | 4 days | Cytotoxicity against human A2780 cells after 4 days by MTT assay, IC50=0.2μM. | 18554906 | ||
| BXPC3 | Cytotoxicity assay | 4 days | Cytotoxicity against human BXPC3 cells after 4 days by MTT assay, IC50=0.24μM. | 18554906 | ||
| NCI446 | Cytotoxicity assay | 4 days | Cytotoxicity against human NCI446 cells after 4 days by MTT assay, IC50=3.28μM. | 18554906 | ||
| HOP62 | Antiproliferative assay | Antiproliferative activity against human HOP62 cells, GI50=0.01μM. | 18630891 | |||
| SF539 | Antiproliferative assay | Antiproliferative activity against human SF539 cells, GI50=0.01μM. | 18630891 | |||
| UACC62 | Antiproliferative assay | Antiproliferative activity against human UACC62 cells, GI50=0.01μM. | 18630891 | |||
| DU145 | Antiproliferative assay | Antiproliferative activity against human DU145 cells, GI50=0.01μM. | 18630891 | |||
| SN12C | Antiproliferative assay | Antiproliferative activity against human SN12C cells, GI50=0.02μM. | 18630891 | |||
| HCT116 | Antiproliferative assay | Antiproliferative activity against human HCT116 cells, GI50=0.03μM. | 18630891 | |||
| MDA-MB-435 | Antiproliferative assay | Antiproliferative activity against human MDA-MB-435 cells, GI50=0.04μM. | 18630891 | |||
| OVCAR-3 | Antiproliferative assay | Antiproliferative activity against human OVCAR-3 cells, GI50=0.22μM. | 18630891 | |||
| RPMI8402 | Cytotoxicity assay | 4 days | Cytotoxicity against human RPMI8402 cells after 4 days by MTT assay, IC50=0.005μM. | 18676151 | ||
| P388 | Cytotoxicity assay | 4 days | Cytotoxicity against mouse P388 cells after 4 days by MTT assay, IC50=0.009μM. | 18676151 | ||
| KB3-1 | Cytotoxicity assay | 4 days | Cytotoxicity against human KB3-1 cells after 4 days by MTT assay, IC50=0.015μM. | 18676151 | ||
| KBV1 | Cytotoxicity assay | 4 days | Cytotoxicity against human multidrug resistant MDR1 overexpressing human KBV1 cells after 4 days by MTT assay, IC50=0.025μM. | 18676151 | ||
| KBH5.0 | Cytotoxicity assay | 4 days | Cytotoxicity against human multidrug resistant BCRP overexpressing human KBH5.0 cells after 4 days by MTT assay, IC50=0.026μM. | 18676151 | ||
| P388 | Cytotoxicity assay | Cytotoxicity against mouse P388 cells by MTT method, IC50=0.004μM. | 18771930 | |||
| RPMI8402 | Cytotoxicity assay | Cytotoxicity against human RPMI8402 cells by MTT method, IC50=0.004μM. | 18771930 | |||
| KB3-1 | Cytotoxicity assay | Cytotoxicity against human KB3-1 cells by MTT method, IC50=0.015μM. | 18771930 | |||
| KBV1 | Cytotoxicity assay | Cytotoxicity against human KBV1 cells by MTT method, IC50=0.025μM. | 18771930 | |||
| KBH5.0 | Cytotoxicity assay | Cytotoxicity against human KBH5.0 cells by MTT method, IC50=0.026μM. | 18771930 | |||
| RPMI8402 | Cytotoxicity assay | Cytotoxicity against human RPMI8402 cells after MTT assay, IC50=0.006μM. | 18829334 | |||
| P388 | Cytotoxicity assay | Cytotoxicity against mouse P388 cells after MTT assay, IC50=0.014μM. | 18829334 | |||
| HEPG2 | Cytotoxicity assay | Cytotoxicity against human HEPG2 cells by MTT assay, IC50=0.3μM. | 18841903 | |||
| HT29 | Cytotoxicity assay | Cytotoxicity against human HT29 cells by MTT assay, IC50=2μM. | 18841903 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by MTT assay, IC50=10μM. | 18841903 | |||
| RPMI8226 | Cytotoxicity assay | Cytotoxicity against human RPMI8226 cells by MTT method, IC50=0.006μM. | 19012996 | |||
| P388 | Cytotoxicity assay | Cytotoxicity against mouse P388 cells by MTT method, IC50=0.014μM. | 19012996 | |||
| CCRF-CEM | Cytotoxicity assay | 3 days | Cytotoxicity against p38-proficient human CCRF-CEM cells after 3 days by MTT assay, IC50=0.04μM. | 19203291 | ||
| A2780 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A2780 cells after 96 hrs by MTT assay, IC50=0.3μM. | 19256478 | ||
| A549 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A549 cells after 96 hrs by MTT assay, IC50=3.1μM. | 19256478 | ||
| HCT8 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HCT8 cells after 96 hrs by MTT assay, IC50=3.2μM. | 19256478 | ||
| BGC823 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human BGC823 cells after 96 hrs by MTT assay, IC50=9.7μM. | 19256478 | ||
| Bel7402 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human Bel7402 cells after 96 hrs by MTT assay, IC50=12.5μM. | 19256478 | ||
| P388 | Cytotoxicity assay | 4 days | Cytotoxicity against mouse P388 cells after 4 days by MTT assay, IC50=0.004μM. | 19299037 | ||
| RPMI8402 | Cytotoxicity assay | 4 days | Cytotoxicity against human RPMI8402 cells after 4 days by MTT assay, IC50=0.004μM. | 19299037 | ||
| P388 | Cytotoxicity assay | 4 days | Cytotoxicity against mouse P388 cells after 4 days by MTT method, IC50=0.004μM. | 19303306 | ||
| RPMI8402 | Cytotoxicity assay | 4 days | Cytotoxicity against human RPMI8402 cells after 4 days by MTT method, IC50=0.004μM. | 19303306 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by sulforhodamine B assay, IC50=0.058μM. | 19345580 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by sulforhodamine B assay, IC50=0.072μM. | 19345580 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells by sulforhodamine B assay, IC50=0.089μM. | 19345580 | |||
| A2780 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A2780 cells after 96 hrs by MTT assay, IC50=0.28μM. | 19453174 | ||
| NCI-H460 | Antiproliferative assay | 1 hr | Antiproliferative activity against human NCI-H460 cells after short term exposure for 1 hr measured after 72 hrs in drug-free medium, IC50=0.33μM. | 19530720 | ||
| A549/ATCC | Antitumor assay | Antitumor activity against human A549/ATCC cells by SRB method, IC50=0.047μM. | 19541483 | |||
| HT-29 | Antitumor assay | Antitumor activity against human HT-29 cells by SRB method, IC50=0.12μM. | 19541483 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells by rapid colorimetric assay, IC50=0.8μM. | 19585998 | |||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells by SRB staining-based fluorescence cytometry, IC50=1.22μM. | 19653666 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by SRB staining-based fluorescence cytometry, IC50=1.22μM. | 19653666 | |||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 cells by SRB staining-based fluorescence cytometry, IC50=1.22μM. | 19653666 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by SRB staining-based fluorescence cytometry, IC50=1.22μM. | 19653666 | |||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTS assay, IC50=0.002μM. | 19655762 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells expressing P-glycoprotein after 72 hrs by MTS assay, IC50=0.003μM. | 19655762 | ||
| HCT15 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT15 cells after 72 hrs by MTS assay, IC50=0.003μM. | 19655762 | ||
| HL60 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL60 cells after 72 hrs by MTS assay, IC50=0.006μM. | 19655762 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTS assay, IC50=0.01μM. | 19655762 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by MTS assay, IC50=0.038μM. | 19655762 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTS assay, IC50=0.455μM. | 19655762 | ||
| SW1573 | Growth inhibition assay | 48 hrs | Growth inhibition of human SW1573 cells after 48 hrs by sulforhodamine B assay, GI50=0.21μM. | 19664930 | ||
| HBL100 | Growth inhibition assay | 48 hrs | Growth inhibition of human HBL100 cells after 48 hrs by sulforhodamine B assay, GI50=0.23μM. | 19664930 | ||
| A2780 | Growth inhibition assay | 48 hrs | Growth inhibition of human A2780 cells after 48 hrs by sulforhodamine B assay, GI50=0.46μM. | 19664930 | ||
| HeLa | Growth inhibition assay | 48 hrs | Growth inhibition of human HeLa cells after 48 hrs by sulforhodamine B assay, GI50=1.3μM. | 19664930 | ||
| WiDr | Growth inhibition assay | 48 hrs | Growth inhibition of human WiDr cells after 48 hrs by sulforhodamine B assay, GI50=1.7μM. | 19664930 | ||
| T47D | Growth inhibition assay | 48 hrs | Growth inhibition of human T47D cells after 48 hrs by sulforhodamine B assay, GI50=1.9μM. | 19664930 | ||
| HCT116 | Antiproliferative assay | 3 days | Antiproliferative activity against human HCT116 cells after 3 days by XTT assay, IC50=0.13μM. | 19691293 | ||
| SW948 | Antiproliferative assay | 3 days | Antiproliferative activity against human SW948 cells after 3 days by XTT assay, IC50=0.16μM. | 19691293 | ||
| A2780 | Cytotoxicity assay | 4 days | Cytotoxicity against human A2780 cells after 4 days by MTT assay, IC50=0.3μM. | 19702283 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells expressing wild type p53 after 48 hrs by SRB assay, GI50=0.01μM. | 19715319 | ||
| AGS | Cytotoxicity assay | 48 hrs | Cytotoxicity against human AGS cells expressing wild type p53 after 48 hrs by SRB assay, GI50=0.01μM. | 19715319 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against estrogen receptor-positive human MCF7 cells expressing wild type p53 after 48 hrs by SRB assay, GI50=0.01μM. | 19715319 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxicity against p53-deficient human Hep3B cells after 48 hrs by SRB assay, GI50=0.01μM. | 19715319 | ||
| AGS | Cytotoxicity assay | 48 hrs | Cytotoxicity against human AGS cells expressing wild type p53 after 48 hrs by SRB assay, IC50=0.02μM. | 19715319 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells expressing p53 mutant after 48 hrs by SRB assay, GI50=0.03μM. | 19715319 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells expressing wild type p53 after 48 hrs by SRB assay, IC50=0.04μM. | 19715319 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against p53-deficient human HeLa cells after 48 hrs by SRB assay, GI50=0.04μM. | 19715319 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxicity against p53-deficient human Hep3B cells after 48 hrs by SRB assay, IC50=0.06μM. | 19715319 | ||
| MDA-MB-261 | Cytotoxicity assay | 48 hrs | Cytotoxicity against estrogen receptor-negative human MDA-MB-261 cells expressing p53 mutant after 48 hrs by SRB assay, GI50=0.08μM. | 19715319 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells expressing wild type p53 after 48 hrs by SRB assay, GI50=0.1μM. | 19715319 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells expressing wild type p53 after 48 hrs by SRB assay, IC50=0.2μM. | 19715319 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against p53-deficient human HeLa cells after 48 hrs by SRB assay, IC50=0.2μM. | 19715319 | ||
| U937 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U937 cells expressing p53 mutant after 48 hrs by SRB assay, IC50=0.3μM. | 19715319 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells expressing p53 mutant after 48 hrs by SRB assay, IC50=0.4μM. | 19715319 | ||
| THP1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human THP1 cells expressing p53 mutant after 48 hrs by SRB assay, IC50=0.6μM. | 19715319 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against p53-deficient human PC3 cells after 48 hrs by SRB assay, GI50=0.7μM. | 19715319 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against estrogen receptor-positive human MCF7 cells expressing wild type p53 after 48 hrs by SRB assay, IC50=1.1μM. | 19715319 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against p53-deficient human PC3 cells after 48 hrs by SRB assay, IC50=1.4μM. | 19715319 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against p53-deficient human K562 cells after 48 hrs by SRB assay, IC50=1.4μM. | 19715319 | ||
| MDA-MB-261 | Cytotoxicity assay | 48 hrs | Cytotoxicity against estrogen receptor-negative human MDA-MB-261 cells expressing p53 mutant after 48 hrs by SRB assay, IC50=1.7μM. | 19715319 | ||
| HT-29 | Cell cycle assay | 0.1 uM | 6 hrs | Cell cycle distribution in human HT-29 cells assessed as accumulation at S phase at 0.1 uM after 6 hrs by flow cytometry | 19715319 | |
| HT-29 | Cell cycle assay | 0.1 uM | 12 hrs | Cell cycle distribution in human HT-29 cells assessed as accumulation at S phase at 0.1 uM after 12 hrs by flow cytometry | 19715319 | |
| HT-29 | Cell cycle assay | 0.1 uM | 24 hrs | Cell cycle distribution in human HT-29 cells assessed as accumulation at S phase at 0.1 uM after 24 hrs by flow cytometry | 19715319 | |
| HT-29 | Cell cycle assay | 0.1 uM | 48 hrs | Cell cycle distribution in human HT-29 cells assessed as accumulation at S phase at 0.1 uM after 48 hrs by flow cytometry | 19715319 | |
| HT-29 | Cell cycle assay | 0.1 uM | 48 hrs | Cell cycle distribution in human HT-29 cells assessed as accumulation at sub-G1 phase at 0.1 uM after 48 hrs by flow cytometry | 19715319 | |
| CCRF-CEM | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CCRF-CEM cells after 48 hrs by cell titer-blue assay, GI50=0.000023μM. | 19743858 | ||
| CCRF-CEM/C2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CCRF-CEM/C2 cells after 48 hrs by cell titer-blue assay, GI50=0.04321μM. | 19743858 | ||
| HOP62 | Cytotoxicity assay | Cytotoxicity against human HOP62 cells, GI50=0.01μM. | 19783447 | |||
| UACC62 | Cytotoxicity assay | Cytotoxicity against human UACC62 cells, GI50=0.01μM. | 19783447 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, GI50=0.01μM. | 19783447 | |||
| SF539 | Cytotoxicity assay | Cytotoxicity against human SF539 cells, GI50=0.01μM. | 19783447 | |||
| SN12C | Cytotoxicity assay | Cytotoxicity against human SN12C cells, GI50=0.02μM. | 19783447 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, GI50=0.03μM. | 19783447 | |||
| MDA-MB-435 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-435 cells, GI50=0.04μM. | 19783447 | |||
| OVCAR-3 | Cytotoxicity assay | Cytotoxicity against human OVCAR-3 cells, GI50=0.22μM. | 19783447 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells by MTT assay, IC50=0.067μM. | 19836231 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by MTT assay, IC50=0.58μM. | 19836231 | |||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells by MTT assay, IC50=0.68μM. | 19836231 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by MTT assay, IC50=1μM. | 19836231 | |||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 cells by MTT assay, IC50=1.07μM. | 19836231 | |||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 cells, ED50=0.06μM. | 19839614 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells by MTT assay, IC50=0.06μM. | 19863101 | |||
| BGC823 | Cytotoxicity assay | Cytotoxicity against human BGC823 cells, IC50=0.21μM. | 19916529 | |||
| A2780 | Cytotoxicity assay | Cytotoxicity against human A2780 cells, IC50=0.28μM. | 19916529 | |||
| MCF7 | Cytotoxicity assay | 4 days | Cytotoxicity against human MCF7 cells after 4 days by ELISA reader assay, IC50=0.06μM. | 19939682 | ||
| K562 | Cytotoxicity assay | 4 days | Cytotoxicity against human K562 cells after 4 days by ELISA reader assay, IC50=0.08μM. | 19939682 | ||
| HeLa | Cytotoxicity assay | 4 days | Cytotoxicity against human HeLa cells after 4 days by ELISA reader assay, IC50=0.63μM. | 19939682 | ||
| DU145 | Cytotoxicity assay | 4 days | Cytotoxicity against human DU145 cells after 4 days by ELISA reader assay, IC50=0.66μM. | 19939682 | ||
| HCT15 | Cytotoxicity assay | 4 days | Cytotoxicity against human HCT15 cells after 4 days by ELISA reader assay, IC50=0.76μM. | 19939682 | ||
| MDA-MB-231 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-231 cells after 3 days, IC50=1.1μM. | 19954977 | ||
| DU145 | Cytotoxicity assay | 3 days | Cytotoxicity against human DU145 cells after 3 days, IC50=1.46μM. | 19954977 | ||
| HeLa | Cytotoxicity assay | 3 days | Cytotoxicity against human HeLa cells after 3 days, IC50=1.85μM. | 19954977 | ||
| HCT116 | Cytotoxicity assay | 3 days | Cytotoxicity against human HCT116 cells after 3 days, IC50=2.77μM. | 19954977 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells after 3 days, IC50=7.42μM. | 19954977 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT method, IC50=0.005379μM. | 20063889 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50=0.065μM. | 20093033 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells after 48 hrs by MTT assay, IC50=0.85μM. | 20093033 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50=0.86μM. | 20093033 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50=0.92μM. | 20093033 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells after 48 hrs by MTT assay, IC50=3.57μM. | 20093033 | ||
| SF539 | Antiproliferative assay | Antiproliferative activity against human SF539 cells, GI50=0.01μM. | 20155916 | |||
| HOP62 | Antiproliferative assay | Antiproliferative activity against human HOP62 cells, GI50=0.01μM. | 20155916 | |||
| DU145 | Antiproliferative assay | Antiproliferative activity against human DU145 cells, GI50=0.01μM. | 20155916 | |||
| UACC62 | Antiproliferative assay | Antiproliferative activity against human UACC62 cells, GI50=0.01μM. | 20155916 | |||
| SN12C | Antiproliferative assay | Antiproliferative activity against human SN12C cells, GI50=0.02μM. | 20155916 | |||
| HCT116 | Antiproliferative assay | Antiproliferative activity against human HCT116 cells, GI50=0.03μM. | 20155916 | |||
| MDA-MB-435 | Antiproliferative assay | Antiproliferative activity against human MDA-MB-435 cells, GI50=0.04μM. | 20155916 | |||
| OVCAR-3 | Antiproliferative assay | Antiproliferative activity against human OVCAR-3 cells, GI50=0.22μM. | 20155916 | |||
| K562 | Cytotoxicity assay | Cytotoxicity against human K562 cells in presence of 10% fetal bovine serum, IC50=0.08μM. | 20188578 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells in presence of 10% fetal bovine serum, IC50=0.63μM. | 20188578 | |||
| HCT15 | Cytotoxicity assay | Cytotoxicity against human HCT15 cells in presence of 10% fetal bovine serum, IC50=0.64μM. | 20188578 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells in presence of 10% fetal bovine serum, IC50=0.94μM. | 20188578 | |||
| THP1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human THP1 cells after 72 hrs by erythosin B staining method, EC50=0.16μM. | 20192247 | ||
| K562 | Cytotoxicity assay | Cytotoxicity against human imitinib-resistant K562 cells by MTT assay, IC50=0.002μM. | 20329739 | |||
| DA1-3b | Cytotoxicity assay | Cytotoxicity against mouse dasatinib-resistant DA1-3b cells carrying pCDNA3-p210BCR/ABL plasmid by MTT assay, IC50=0.03μM. | 20329739 | |||
| Raji | Cytotoxicity assay | Cytotoxicity against human Raji cells by MTT assay, IC50=1μM. | 20329739 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells by MTT assay, IC50=0.59μM. | 20356735 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by MTT assay, IC50=0.94μM. | 20356735 | |||
| KB | Cytotoxicity assay | 3 days | Cytotoxicity against human KB cells after 3 days by MTT assay, IC50<0.013μM. | 20392545 | ||
| HCT8 | Cytotoxicity assay | 3 days | Cytotoxicity against human HCT8 cells after 3 days by MTT assay, IC50<0.013μM. | 20392545 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells after 3 days by MTT assay, IC50=0.02μM. | 20392545 | ||
| HL60 | Cytotoxicity assay | 2 days | Cytotoxicity against human HL60 cells after 2 days by automatic ELISA reader system, IC50=0.1μM. | 20392646 | ||
| MDA-MB-231 | Cytotoxicity assay | 2 days | Cytotoxicity against human MDA-MB-231 cells after 2 days by automatic ELISA reader system, IC50=0.3μM. | 20392646 | ||
| HeLa | Cytotoxicity assay | 2 days | Cytotoxicity against human HeLa cells after 2 days by automatic ELISA reader system, IC50=0.4μM. | 20392646 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days by automatic ELISA reader system, IC50=0.4μM. | 20392646 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days by automatic ELISA reader system, IC50=0.5μM. | 20392646 | ||
| SKBR3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against estrogen receptor deficient human SKBR3 cells after 48 hrs by SRB assay, GI50=0.15μM. | 20438092 | ||
| MCF7/BUS | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7/BUS cells expressing estrogen receptor after 48 hrs by SRB assay, GI50=0.21μM. | 20438092 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells expressing estrogen receptor after 48 hrs by SRB assay, GI50=0.28μM. | 20438092 | ||
| MDA-MB-231 | Antiproliferative assay | 48 hrs | Antiproliferative activity against estrogen receptor deficient human MDA-MB-231 cells after 48 hrs by SRB assay, GI50=1.3μM. | 20438092 | ||
| T47D | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T47D cells expressing estrogen receptor after 48 hrs by SRB assay, GI50=2μM. | 20438092 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against imatinib-resistant human K562 cells after 72 hrs by MTT assay, IC50=0.002μM. | 20590148 | ||
| DA1-3b/M2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against imatinib and dasatinib-resistant mouse DA1-3b/M2 cells after 72 hrs by MTT assay, IC50=0.03μM. | 20590148 | ||
| NCI-H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H460 cells after 72 hrs by XTT assay, GI50=0.03μM. | 20591678 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by XTT assay, GI50=0.06μM. | 20591678 | ||
| SF268 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SF268 cells after 72 hrs by XTT assay, GI50=0.19μM. | 20591678 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by XTT assay, GI50=0.57μM. | 20591678 | ||
| Detroit 551 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Detroit 551 cells after 72 hrs by XTT assay, GI50=0.99μM. | 20591678 | ||
| HeLa | Cytotoxicity assay | 4 days | Cytotoxicity against human HeLa cells after 4 days by MTT assay, IC50=1.1μM. | 20619511 | ||
| HCT116 | Cytotoxicity assay | 4 days | Cytotoxicity against human HCT116 cells after 4 days by MTT assay, IC50=2.3μM. | 20619511 | ||
| DU145 | Cytotoxicity assay | 4 days | Cytotoxicity against human DU145 cells after 4 days by MTT assay, IC50=3.2μM. | 20619511 | ||
| MDA-MB-231 | Cytotoxicity assay | 4 days | Cytotoxicity against human MDA-MB-231 cells after 4 days by MTT assay, IC50=4.2μM. | 20619511 | ||
| MCF7 | Cytotoxicity assay | 4 days | Cytotoxicity against human MCF7 cells after 4 days by MTT assay, IC50=14.2μM. | 20619511 | ||
| SF539 | Cytotoxicity assay | Cytotoxicity against human SF539 cells, GI50=0.01μM. | 20630766 | |||
| UACC62 | Cytotoxicity assay | Cytotoxicity against human UACC62 cells, GI50=0.01μM. | 20630766 | |||
| HOP62 | Cytotoxicity assay | Cytotoxicity against human HOP62 cells, GI50=0.01μM. | 20630766 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, GI50=0.01μM. | 20630766 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, GI50=0.01μM. | 20630766 | |||
| SN12C | Cytotoxicity assay | Cytotoxicity against human SN12C cells, GI50=0.02μM. | 20630766 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, GI50=0.03μM. | 20630766 | |||
| OVCAR-3 | Cytotoxicity assay | Cytotoxicity against human OVCAR-3 cells, GI50=0.22μM. | 20630766 | |||
| U87MG | Cytotoxicity assay | 6 days | Cytotoxicity against human U87MG cells after 6 days, LD50=0.02μM. | 20655220 | ||
| U87MG | Cytotoxicity assay | 250 nM | 6 days | Cytotoxicity against human U87MG cells at 250 nM after 6 days | 20655220 | |
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, GI50=0.08μM. | 20662543 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs by MTT assay, GI50=0.09μM. | 20662543 | ||
| SKHep | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKHep cells after 48 hrs by MTT assay, GI50=0.22μM. | 20662543 | ||
| Detroit 551 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Detroit 551 cells after 48 hrs by MTT assay, GI50=0.99μM. | 20662543 | ||
| BT-483 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human BT-483 cells after 48 hrs by MTT assay, GI50=1.03μM. | 20662543 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, GI50=2.8μM. | 20662543 | ||
| SAS | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SAS cells after 48 hrs by MTT assay, GI50=6μM. | 20662543 | ||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 cells, EC50=0.06μM. | 20825206 | |||
| HT-29 | Cytotoxicity assay | 3 days | Cytotoxicity against human HT-29 cells after 3 days by sulforhodamine B assay, ED50=0.06μM. | 20939540 | ||
| A549 | Cytotoxicity assay | 3 days | Cytotoxicity against human A549 cells after 3 days by MTT assay, IC50=0.057μM. | 20942490 | ||
| MDA-MB-435 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-435 cells after 3 days by MTT assay, IC50=0.71μM. | 20942490 | ||
| LoVo | Cytotoxicity assay | 3 days | Cytotoxicity against human LoVo cells after 3 days by MTT assay, IC50=3.1μM. | 20942490 | ||
| A2780 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A2780 cells after 24 hrs by MTT assay, IC50=0.2μM. | 20961093 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells after 24 hrs by MTT assay, IC50=3.6μM. | 20961093 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by trypan blue assay, IC50=0.0089μM. | 21071230 | ||
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells after 72 hrs by trypan blue assay, IC50=0.011μM. | 21071230 | ||
| HL60 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL60 cells after 72 hrs by trypan blue assay, IC50=0.017μM. | 21071230 | ||
| DU145 | Anticancer assay | 48 hrs | Anticancer activity against human DU145 cells assessed as cell viability after 48 hrs by SRB assay, IC50=0.02μM. | 21074444 | ||
| A549 | Anticancer assay | 48 hrs | Anticancer activity against human A549 cells assessed as cell viability after 48 hrs by SRB assay, IC50=0.03μM. | 21074444 | ||
| PC3 | Anticancer assay | 48 hrs | Anticancer activity against human PC3 cells assessed as cell viability after 48 hrs by SRB assay, IC50=0.03μM. | 21074444 | ||
| HCT15 | Anticancer assay | 48 hrs | Anticancer activity against human HCT15 cells assessed as cell viability after 48 hrs by SRB assay, IC50=0.067μM. | 21074444 | ||
| IMR32 | Anticancer assay | 48 hrs | Anticancer activity against human IMR32 cells assessed as cell viability after 48 hrs by SRB assay, IC50=0.08μM. | 21074444 | ||
| Hep2 | Anticancer assay | 48 hrs | Anticancer activity against human Hep2 cells assessed as cell viability after 48 hrs by SRB assay, IC50=0.7μM. | 21074444 | ||
| MOLT4 | Cell cycle assay | 5 uM | 24 hrs | Cell cycle arrest in human MOLT4 cells assessed as accumulation at sub G1 phase at 5 uM after 24 hrs using propidium iodide staining by flow cytometry | 21074444 | |
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells, IC50=0.018μM. | 21095131 | |||
| Col2 | Cytotoxicity assay | Cytotoxicity against human Col2 cells, IC50=0.045μM. | 21095131 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells, IC50=0.069μM. | 21095131 | |||
| HT1080 | Cytotoxicity assay | Cytotoxicity against human HT1080 cells, IC50=0.08μM. | 21095131 | |||
| SNU638 | Cytotoxicity assay | Cytotoxicity against human SNU638 cells, IC50=0.098μM. | 21095131 | |||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, GI50=0.01μM. | 21115210 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by MTT assay, GI50=0.05μM. | 21115210 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by MTT assay, GI50=0.06μM. | 21115210 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by MTT assay, GI50=0.06μM. | 21115210 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, TGI=0.16μM. | 21115210 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by MTT assay, LC50=31.62μM. | 21115210 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by MTT assay, TGI=31.62μM. | 21115210 | ||
| K562 | Cytotoxicity assay | Cytotoxicity against human K562 cells by MTT assay, IC50=0.59μM. | 21115246 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by MTT assay, IC50=0.61μM. | 21115246 | |||
| HCT15 | Cytotoxicity assay | Cytotoxicity against human HCT15 cells by MTT assay, IC50=0.8μM. | 21115246 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by MTT assay, IC50=2.43μM. | 21115246 | |||
| U87 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87 cells after 72 hrs by MTT assay, IC50=0.5μM. | 21186067 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50=0.65μM. | 21186067 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50=1.5μM. | 21186067 | ||
| CHO | Function assay | 10 uM | 30 mins | Drug uptake in CHO cells at 10 uM after 30 mins by confocal microscopy | 21282053 | |
| CHO | Function assay | 10 uM | 30 mins | Drug uptake in NK1R overexpressing CHO cells at 10 uM after 30 mins by confocal microscopy | 21282053 | |
| HL60 | Apoptosis assay | 4 uM | 9 hrs | Induction of apoptosis in human HL60 cells assessed as DNA ladder formation at 4 uM after 9 hrs by agarose gel electrophoresis | 21334793 | |
| H69 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H69 cells after 72 hrs by alamar blue assay, IC50=0.001μM. | 21341674 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by alamar blue assay, IC50=0.012μM. | 21341674 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs by alamar blue assay, IC50=0.013μM. | 21341674 | ||
| MES-SA/Dx5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MES-SA/Dx5 cells overexpressing MDR1 after 72 hrs by alamar blue assay, IC50=0.052μM. | 21341674 | ||
| MESSA | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MESSA cells after 72 hrs by alamar blue assay, IC50=0.057μM. | 21341674 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by alamar blue assay, IC50=0.26μM. | 21341674 | ||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells by MTT assay, IC50=0.018μM. | 21353568 | |||
| Col2 | Cytotoxicity assay | Cytotoxicity against human Col2 cells by MTT assay, IC50=0.045μM. | 21353568 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50=0.069μM. | 21353568 | |||
| HT1080 | Cytotoxicity assay | Cytotoxicity against human HT1080 cells by MTT assay, IC50=0.08μM. | 21353568 | |||
| SNU638 | Cytotoxicity assay | Cytotoxicity against human SNU638 cells by MTT assay, IC50=0.098μM. | 21353568 | |||
| LoVo | Cytotoxicity assay | 72 hrs | Cytotoxicity against human LoVo cells after 72 hrs by MTT assay, GI50=0.008μM. | 21388138 | ||
| U373 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U373 cells after 72 hrs by MTT assay, GI50=0.033μM. | 21388138 | ||
| SK-MEL | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SK-MEL cells after 72 hrs by MTT assay, GI50=0.047μM. | 21388138 | ||
| Jurkat | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Jurkat cells after 72 hrs by MTT assay, GI50=0.09μM. | 21388138 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, GI50=0.2μM. | 21388138 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by MTT assay, GI50=0.48μM. | 21388138 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, GI50=1.3μM. | 21388138 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, GI50=6μM. | 21388138 | ||
| MDA-MB-231 | Cell cycle assay | 24 hrs | Cell cycle arrest in human MDA-MB-231 cells assessed as increase of cells in sub-G0 phase after 24 hrs using annexin V/propidium iodide staining by flow cytometry | 21391655 | ||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by SRB assay, IC50=0.0016μM. | 21410162 | |||
| HL60 | Cytotoxicity assay | 2 days | Cytotoxicity against human HL60 cells after 2 days, IC50=0.065μM. | 21419530 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days, IC50=0.31μM. | 21419530 | ||
| MDA-MB-231 | Cytotoxicity assay | 2 days | Cytotoxicity against human MDA-MB-231 cells after 2 days, IC50=0.97μM. | 21419530 | ||
| HeLa | Cytotoxicity assay | 2 days | Cytotoxicity against human HeLa cells after 2 days, IC50=1.08μM. | 21419530 | ||
| HCT116 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT116 cells after 2 days, IC50=3.97μM. | 21419530 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs by MTS assay, IC50<0.04μM. | 21513293 | ||
| SF268 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SF268 cells after 72 hrs by MTS assay, IC50=0.04μM. | 21513293 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTS assay, IC50=0.07μM. | 21513293 | ||
| NCI-H460 | Anticancer assay | Anticancer activity against human NCI-H460 cells, IC50=0.03μM. | 21599000 | |||
| SF268 | Anticancer assay | Anticancer activity against human SF268 cells, IC50=0.19μM. | 21599000 | |||
| MRC5 | Antiproliferative assay | Antiproliferative activity against human MRC5 cells, IC50=0.89μM. | 21599000 | |||
| MCF7 | Anticancer assay | Anticancer activity against human MCF7 cells, IC50=11.12μM. | 21599000 | |||
| HL60 | Cytotoxicity assay | 2 days | Cytotoxicity against human HL60 cells after 2 days, IC50=0.1μM. | 21601964 | ||
| MDA-MB-231 | Cytotoxicity assay | 2 days | Cytotoxicity against human MDA-MB-231 cells after 2 days, INH=0.3μM. | 21601964 | ||
| HeLa | Cytotoxicity assay | 2 days | Cytotoxicity against human HeLa cells after 2 days, IC50=0.4μM. | 21601964 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days, IC50=0.4μM. | 21601964 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days, IC50=0.5μM. | 21601964 | ||
| SW620 | Apoptosis assay | 5 uM | 6 hrs | Induction of apoptosis in human SW620 cells assessed as DNA fragmentation at 5 uM after 6 hrs by gel electrophoresis | 21620534 | |
| HOP62 | Antiproliferative assay | Antiproliferative activity against human HOP62 cells by SRB assay, GI50=0.01μM. | 21710981 | |||
| SF539 | Antiproliferative assay | Antiproliferative activity against human SF539 cells by SRB assay, GI50=0.01μM. | 21710981 | |||
| UACC62 | Antiproliferative assay | Antiproliferative activity against human UACC62 cells by SRB assay, GI50=0.01μM. | 21710981 | |||
| DU145 | Antiproliferative assay | Antiproliferative activity against human DU145 cells by SRB assay, GI50=0.01μM. | 21710981 | |||
| MCF7 | Antiproliferative assay | Antiproliferative activity against human MCF7 cells by SRB assay, GI50=0.01μM. | 21710981 | |||
| SN12C | Antiproliferative assay | Antiproliferative activity against human SN12C cells by SRB assay, GI50=0.02μM. | 21710981 | |||
| HCT116 | Antiproliferative assay | Antiproliferative activity against human HCT116 cells by SRB assay, GI50=0.03μM. | 21710981 | |||
| OVCAR3 | Antiproliferative assay | Antiproliferative activity against human OVCAR3 cells by SRB assay, GI50=0.22μM. | 21710981 | |||
| MIAPaCa2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MIAPaCa2 cells after 24 hrs by MTT assay, IC50=0.4μM. | 21775156 | ||
| HOP62 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HOP62 cells after 48 hrs by SRB assay, GI50=0.01μM. | 21823606 | ||
| UACC62 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human UACC62 cells after 48 hrs by SRB assay, GI50=0.01μM. | 21823606 | ||
| SF539 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SF539 cells after 48 hrs by SRB assay, GI50=0.01μM. | 21823606 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells after 48 hrs by SRB assay, GI50=0.01μM. | 21823606 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by SRB assay, GI50=0.013μM. | 21823606 | ||
| SN12C | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SN12C cells after 48 hrs by SRB assay, GI50=0.02μM. | 21823606 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by SRB assay, GI50=0.03μM. | 21823606 | ||
| OVCAR3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human OVCAR3 cells after 48 hrs by SRB assay, GI50=0.22μM. | 21823606 | ||
| Caco2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human Caco2 cells assessed as cell viability after 24 hrs by MTT assay, IC50=9.55μM. | 21843907 | ||
| HT-29 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HT-29 cells assessed as cell viability after 24 hrs by MTT assay, IC50=10μM. | 21843907 | ||
| HT-29 | Function assay | 24 hrs | Induction of mitochondrial dysfunction in human HT-29 cells assessed as increase in cytosolic cytochrome c level after 24 hrs by enzyme immunometric assay | 21843907 | ||
| Caco2 | Function assay | 24 hrs | Induction of mitochondrial dysfunction in human Caco2 cells assessed as increase in cytosolic cytochrome c level after 24 hrs by enzyme immunometric assay | 21843907 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50=0.091μM. | 21873069 | ||
| HCT15 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT15 cells after 48 hrs by MTT assay, IC50=0.166μM. | 21873069 | ||
| SKOV3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKOV3 cells after 48 hrs by MTT assay, IC50=2.544μM. | 21873069 | ||
| SK-MEL-2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SK-MEL-2 cells after 48 hrs by MTT assay, IC50=7.86μM. | 21873069 | ||
| SMMC7721 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SMMC7721 cells after 48 hrs by SRB assay, IC50=0.003μM. | 21973054 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by SRB assay, IC50=0.056μM. | 21978950 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells assessed as inhibition of cell viability after 24 hrs by MTT assay, IC50=8.93μM. | 21983445 | ||
| A2780 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A2780 cells after 96 hrs by MTT assay, IC50=0.26μM. | 22044245 | ||
| HCT8 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HCT8 cells after 96 hrs by MTT assay, IC50=0.26μM. | 22044245 | ||
| Bel7402 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human Bel7402 cells after 96 hrs by MTT assay, IC50=0.26μM. | 22044245 | ||
| BCG823 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human BCG823 cells after 96 hrs by MTT assay, IC50=0.26μM. | 22044245 | ||
| A549 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A549 cells after 96 hrs by MTT assay, IC50=0.26μM. | 22044245 | ||
| CCRF-CEM | Cytotoxicity assay | 96 hrs | Cytotoxicity against human CCRF-CEM cells after 96 hrs by MTT assay, CC50=0.003μM. | 22047799 | ||
| CCRF-SB | Cytotoxicity assay | 96 hrs | Cytotoxicity against human CCRF-SB cells after 96 hrs by MTT assay, CC50=0.004μM. | 22047799 | ||
| WIL2-NS | Cytotoxicity assay | 96 hrs | Cytotoxicity against human WIL2-NS cells after 96 hrs by MTT assay, CC50=0.005μM. | 22047799 | ||
| DU145 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human DU145 cells after 96 hrs by MTT assay, CC50=0.015μM. | 22047799 | ||
| MCF7 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MCF7 cells after 96 hrs by MTT assay, CC50=0.02μM. | 22047799 | ||
| HepG2 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HepG2 cells after 96 hrs by MTT assay, CC50=0.02μM. | 22047799 | ||
| SKMES1 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human SKMES1 cells after 96 hrs by MTT assay, CC50=0.03μM. | 22047799 | ||
| SK-MEL-28 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human SK-MEL-28 cells after 96 hrs by MTT assay, CC50=0.07μM. | 22047799 | ||
| MRC5 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MRC5 cells after 96 hrs by MTT assay, CC50=0.2μM. | 22047799 | ||
| CRL7065 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human CRL7065 cells after 96 hrs by MTT assay, CC50=0.3μM. | 22047799 | ||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human tamoxifen-resistant MCF7 cells by WST-1 assay, IC50=1.8μM. | 22197145 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by WST-1 assay, IC50=2.6μM. | 22197145 | |||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells by WST-1 assay, IC50=2.7μM. | 22197145 | |||
| Jurkat T | Apoptosis assay | 2 uM | 24 hrs | Induction of apoptosis in human Jurkat T cells assessed as increase in phosphatidylserine level at 2 uM after 24 hrs by flow cytometric analysis | 22200402 | |
| MT4 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MT4 cells after 96 hrs, IC50=0.004μM. | 22276775 | ||
| DU145 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human DU145 cells after 96 hrs, IC50=0.015μM. | 22276775 | ||
| HepG2 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HepG2 cells after 96 hrs, IC50=0.02μM. | 22276775 | ||
| CEM | Cytotoxicity assay | Cytotoxicity against human CEM cells, IC50=0.005μM. | 22305342 | |||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days, IC50=0.26μM. | 22318164 | ||
| HeLa | Cytotoxicity assay | 2 days | Cytotoxicity against human HeLa cells after 2 days, IC50=1.01μM. | 22318164 | ||
| K562 | Cytotoxicity assay | 2 days | Cytotoxicity against human K562 cells after 2 days, IC50=1.18μM. | 22318164 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days, IC50=2.37μM. | 22318164 | ||
| MCF7 | Cytotoxicity assay | 2 days | Cytotoxicity against human MCF7 cells after 2 days, IC50=3.91μM. | 22318164 | ||
| A549 | Cytotoxicity assay | 2 days | Cytotoxicity against human A549 cells after 2 days by MTT assay, IC50=0.008μM. | 22325897 | ||
| Jurkat | Cytotoxicity assay | 2 days | Cytotoxicity against human Jurkat cells after 2 days by MTT assay, IC50=0.026μM. | 22325897 | ||
| THP1 | Cytotoxicity assay | 2 days | Cytotoxicity against human THP1 cells after 2 days by MTT assay, IC50=0.071μM. | 22325897 | ||
| HL60 | Cytotoxicity assay | 2 days | Cytotoxicity against human HL60 cells after 2 days by MTT assay, IC50=0.6μM. | 22325897 | ||
| U937 | Cytotoxicity assay | 2 days | Cytotoxicity against human U937 cells after 2 days by MTT assay, IC50=1.98μM. | 22325897 | ||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells after 24 hrs by MTT assay, IC50=6.4μM. | 22326393 | ||
| FHCC-98 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human FHCC-98 cells after 24 hrs by MTT assay, IC50=15.2μM. | 22326393 | ||
| QSG7701 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human QSG7701 cells after 24 hrs by MTT assay, IC50=18.3μM. | 22326393 | ||
| SF539 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF539 cells after 48 hrs by sulforhodamine B assay, GI50=0.01μM. | 22329436 | ||
| UACC62 | Growth inhibition assay | 48 hrs | Growth inhibition of human UACC62 cells after 48 hrs by sulforhodamine B assay, GI50=0.01μM. | 22329436 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by sulforhodamine B assay, GI50=0.01μM. | 22329436 | ||
| HOP62 | Growth inhibition assay | 48 hrs | Growth inhibition of human HOP62 cells after 48 hrs by sulforhodamine B assay, GI50=0.01μM. | 22329436 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by sulforhodamine B assay, GI50=0.013μM. | 22329436 | ||
| SN12C | Growth inhibition assay | 48 hrs | Growth inhibition of human SN12C cells after 48 hrs by sulforhodamine B assay, GI50=0.02μM. | 22329436 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT116 cells after 48 hrs by sulforhodamine B assay, GI50=0.03μM. | 22329436 | ||
| OVCAR3 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR3 cells after 48 hrs by sulforhodamine B assay, GI50=0.22μM. | 22329436 | ||
| COLO205 | Apoptosis assay | 4 uM | 24 hrs | Induction of apoptosis in human COLO205 cells assessed as down regulation of Bcl-2 at 4 uM after 24 hrs by ELISA | 22364746 | |
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by SRB assay, IC50=0.0016μM. | 22376176 | |||
| HeLa | Function assay | 50 uM | 6 hrs | Inhibition of Mcl-1 in doxycyclin-stimulated human HeLa cells overexpressing Bad3SA assessed as increase in caspase3/7 activity at 50 uM after 6 hrs by luminescence analysis | 22386982 | |
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells after 72 hrs by SRB assay, IC50<0.1μM. | 22409771 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by SRB assay, IC50<0.1μM. | 22409771 | ||
| Mahlavu | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Mahlavu cells after 72 hrs by SRB assay, IC50<0.1μM. | 22409771 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by SRB assay, IC50<0.1μM. | 22409771 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by SRB assay, IC50<0.1μM. | 22409771 | ||
| FOCUS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human FOCUS cells after 72 hrs by SRB assay, IC50<0.1μM. | 22409771 | ||
| HEK293 | Cytotoxicity assay | 2 days | Cytotoxicity against human HEK293 cells after 2 days by cell counting kit-8 analysis, IC50=1.01μM. | 22503656 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days by cell counting kit-8 analysis, IC50=1.06μM. | 22503656 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days by cell counting kit-8 analysis, IC50=1.38μM. | 22503656 | ||
| T47D | Cytotoxicity assay | 2 days | Cytotoxicity against human T47D cells after 2 days by cell counting kit-8 analysis, IC50=1.53μM. | 22503656 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTS assay, IC50=0.4μM. | 22542106 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTS assay, IC50=1.57μM. | 22542106 | ||
| KB-7d | Antiproliferative assay | 72 hrs | Antiproliferative activity against human VP16-resistant KB-7d cells overexpressing MRP after 72 hrs by ethylene blue dye assay, IC50=0.0068μM. | 22698783 | ||
| KB | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KB cells after 72 hrs by ethylene blue dye assay, IC50=0.0088μM. | 22698783 | ||
| KBCPT100 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human partially revertant camptothecin-resistant KBCPT100 cells after 72 hrs by ethylene blue dye assay, IC50=0.024μM. | 22698783 | ||
| KBCPT100 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human camptothecin-resistant KBCPT100 cells after 72 hrs by ethylene blue dye assay, IC50=0.423μM. | 22698783 | ||
| NCI-H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H460 cells after 72 hrs by sulforhodamine B assay, IC50=0.005μM. | 22749423 | ||
| A2780 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A2780 cells after 72 hrs by sulforhodamine B assay, IC50=0.007μM. | 22749423 | ||
| GLC4 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GLC4 cells after 72 hrs by sulforhodamine B assay, IC50=0.01μM. | 22749423 | ||
| NCI-H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H460 cells incubated for 72 hrs by MTT assay, IC50=0.003μM. | 22758788 | ||
| HEK293 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HEK293 cells after 72 hrs by MTT assay, IC50=7.94μM. | 22819942 | ||
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells after 72 hrs by MTT assay, IC50=9.17μM. | 22819942 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by MTT assay, IC50=9.29μM. | 22819942 | ||
| HCT15 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT15 cells after 72 hrs by MTT assay, IC50=9.92μM. | 22819942 | ||
| NCI-H460 | Cytotoxicity assay | 3 days | Cytotoxicity against human NCI-H460 cells incubated for 3 days by SRB assay, IC50=0.002μM. | 22863526 | ||
| SF268 | Cytotoxicity assay | 3 days | Cytotoxicity against human SF268 cells incubated for 3 days by SRB assay, IC50=0.07μM. | 22863526 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells incubated for 3 days by SRB assay, IC50=0.08μM. | 22863526 | ||
| IMR90 | Cytotoxicity assay | 3 days | Cytotoxicity against human IMR90 cells incubated for 3 days by SRB assay, IC50=0.43μM. | 22863526 | ||
| HL60 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL60 cells incubated for 72 hrs by MTT assay, GI50<0.003μM. | 22867019 | ||
| MDA-MB-435 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435 cells incubated for 72 hrs by MTT assay, GI50=0.033μM. | 22867019 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells incubated for 72 hrs by MTT assay, GI50=0.083μM. | 22867019 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells incubated for 72 hrs by MTT assay, GI50=0.17μM. | 22867019 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells incubated for 72 hrs by MTT assay, GI50=0.22μM. | 22867019 | ||
| PANC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PANC1 cells incubated for 72 hrs by MTT assay, GI50=6.29μM. | 22867019 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells incubated for 72 hrs by SRB assay, IC50=0.01μM. | 22867707 | ||
| BT20 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BT20 cells incubated for 72 hrs by SRB assay, IC50=0.07μM. | 22867707 | ||
| CAMA1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CAMA1 cells incubated for 72 hrs by SRB assay, IC50=0.07μM. | 22867707 | ||
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells incubated for 72 hrs by SRB assay, IC50=0.15μM. | 22867707 | ||
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells incubated for 72 hrs by SRB assay, IC50<1μM. | 22867707 | ||
| MFE296 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MFE296 cells incubated for 72 hrs by SRB assay, IC50<1μM. | 22867707 | ||
| MV | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MV cells incubated for 72 hrs by SRB assay, IC50<1μM. | 22867707 | ||
| FOCUS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human FOCUS cells incubated for 72 hrs by SRB assay, IC50<1μM. | 22867707 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells incubated for 72 hrs by SRB assay, IC50<1μM. | 22867707 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells incubated for 72 hrs by SRB assay, IC50<1μM. | 22867707 | ||
| CCRF-CEM | Cytotoxicity assay | 96 hrs | Cytotoxicity against human CCRF-CEM cells after 96 hrs by MTT assay, CC50=0.003μM. | 22884523 | ||
| MT4 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MT4 cells after 96 hrs by MTT assay, CC50=0.004μM. | 22884523 | ||
| CCRF-SB | Cytotoxicity assay | 96 hrs | Cytotoxicity against human CCRF-SB cells after 96 hrs by MTT assay, CC50=0.004μM. | 22884523 | ||
| WIL2-NS | Cytotoxicity assay | 96 hrs | Cytotoxicity against human WIL2-NS cells after 96 hrs by MTT assay, CC50=0.005μM. | 22884523 | ||
| HepG2 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HepG2 cells after 96 hrs by MTT assay, CC50=0.04μM. | 22884523 | ||
| SKMES1 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human SKMES1 cells after 96 hrs by MTT assay, CC50=0.05μM. | 22884523 | ||
| SK-MEL-28 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human SK-MEL-28 cells after 96 hrs by MTT assay, CC50=0.07μM. | 22884523 | ||
| DU145 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human DU145 cells after 96 hrs by MTT assay, CC50=0.08μM. | 22884523 | ||
| MCF7 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MCF7 cells after 96 hrs by MTT assay, CC50=0.08μM. | 22884523 | ||
| MRC5 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MRC5 cells after 96 hrs by MTT assay, CC50=0.2μM. | 22884523 | ||
| CRL7065 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human CRL7065 cells after 96 hrs by MTT assay, CC50=0.3μM. | 22884523 | ||
| MDA-MB-435 | Antiproliferative assay | 3 days | Antiproliferative activity against human MDA-MB-435 cells after 3 days by MTT assay, IC50=0.0436μM. | 23084702 | ||
| HCT116 | Antiproliferative assay | 3 days | Antiproliferative activity against human HCT116 cells after 3 days by MTT assay, IC50=0.11μM. | 23084702 | ||
| A549 | Antiproliferative assay | 3 days | Antiproliferative activity against human A549 cells after 3 days by MTT assay, IC50=2.61μM. | 23084702 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB assay, IC50=0.0158μM. | 23102893 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by SRB assay, IC50=0.0287μM. | 23102893 | |||
| KBVIN | Cytotoxicity assay | Cytotoxicity against human KBVIN cells by SRB assay, IC50=0.0371μM. | 23102893 | |||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells by SRB assay, IC50=0.121μM. | 23102893 | |||
| HCT116 | Cytotoxicity assay | 3 days | Cytotoxicity against human HCT116 cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| SKBR3 | Cytotoxicity assay | 3 days | Cytotoxicity against human SKBR3 cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| ZR-75-1 | Cytotoxicity assay | 3 days | Cytotoxicity against human ZR-75-1 cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| MCF12A | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF12A cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| MDA-MB-453 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-453 cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| BT20 | Cytotoxicity assay | 3 days | Cytotoxicity against human BT20 cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| T47D | Cytotoxicity assay | 3 days | Cytotoxicity against human T47D cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| MDA-MB-231 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-231 cells after 3 days by SRB assay, IC50<0.01μM. | 23142674 | ||
| MDA-MB-157 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-157 cells after 3 days by SRB assay, IC50=0.02μM. | 23142674 | ||
| HuH7 | Cytotoxicity assay | 3 days | Cytotoxicity against human HuH7 cells after 3 days by SRB assay, IC50=0.06μM. | 23142674 | ||
| CAMA1 | Cytotoxicity assay | 3 days | Cytotoxicity against human CAMA1 cells after 3 days by SRB assay, IC50=0.07μM. | 23142674 | ||
| MDA-MB-361 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-361 cells after 3 days by SRB assay, IC50=0.17μM. | 23142674 | ||
| BT474 | Cytotoxicity assay | 3 days | Cytotoxicity against human BT474 cells after 3 days by SRB assay, IC50=12.75μM. | 23142674 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTS assay, IC50=0.4μM. | 23153813 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTS assay, IC50=1.57μM. | 23153813 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by MTT assay, GI50=0.002μM. | 23357037 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition of human A549 cells after 48 hrs by MTT assay, GI50=0.007μM. | 23357037 | ||
| HeLa | Growth inhibition assay | 48 hrs | Growth inhibition of human HeLa cells after 48 hrs by MTT assay, GI50=0.05μM. | 23357037 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by SRB assay, IC50=32.3μM. | 23562244 | ||
| L1210 | Cytotoxicity assay | Cytotoxicity against mouse L1210 cells, IC50=0.03μM. | 23578545 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by WST assay, IC50=0.067μM. | 23578545 | |||
| T24 | Cytotoxicity assay | Cytotoxicity against human chemo-resistant T24 cells by WST assay, IC50=0.088μM. | 23578545 | |||
| HL60 | Apoptosis assay | 5 uM | 6 hrs | Induction of apoptosis in human HL60 cells assessed as DNA ladder formation at 5 uM after 6 hrs by DNA agarose gel electrophoresis | 23584541 | |
| T47D | Cytotoxicity assay | Cytotoxicity against human T47D cells by MTT assay, IC50=0.07μM. | 23608763 | |||
| SNU638 | Cytotoxicity assay | Cytotoxicity against human SNU638 cells by MTT assay, IC50=0.28μM. | 23608763 | |||
| CEM | Growth inhibition assay | Growth inhibition of human CEM cells, GI50=0.002μM. | 23750455 | |||
| CEM/C2 | Growth inhibition assay | Growth inhibition of multidrug-resistant human CEM/C2 cells, GI50=1μM. | 23750455 | |||
| HBL100 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HBL100 cells after 48 hrs by SRB assay, GI50=0.23μM. | 23831507 | ||
| SW1573 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SW1573 cells after 48 hrs by SRB assay, GI50=0.25μM. | 23831507 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by SRB assay, GI50=0.6μM. | 23831507 | ||
| WiDr | Antiproliferative assay | 48 hrs | Antiproliferative activity against human WiDr cells after 48 hrs by SRB assay, GI50=1.8μM. | 23831507 | ||
| T47D | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T47D cells after 48 hrs by SRB assay, GI50=2μM. | 23831507 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, GI50=0.01μM. | 23831811 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by MTT assay, GI50=0.05μM. | 23831811 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, TGI=0.16μM. | 23831811 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by MTT assay, TGI=0.79μM. | 23831811 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by MTT assay, LC50=31.62μM. | 23831811 | ||
| MS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MS cells after 72 hrs ny MTT assay, IC50=0.23μM. | 23974020 | ||
| TE-32 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human TE-32 cells after 72 hrs ny MTT assay, IC50=4.47μM. | 23974020 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs ny MTT assay, IC50=37.05μM. | 23974020 | ||
| T47D | Cytotoxicity assay | 2 days | Cytotoxicity against human T47D cells after 2 days by CCK-8 assay, IC50=0.3μM. | 24013413 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days by CCK-8 assay, IC50=0.39μM. | 24013413 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days by CCK-8 assay, IC50=0.42μM. | 24013413 | ||
| HEK293 | Cytotoxicity assay | 2 days | Cytotoxicity against HEK293 cells after 2 days by CCK-8 assay, IC50=0.78μM. | 24013413 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by WST-8 assay, IC50=0.016μM. | 24033077 | ||
| COLO205 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human COLO205 cells after 48 hrs by MTT assay, IC50=0.05μM. | 24041234 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50=0.06μM. | 24041234 | ||
| MIAPaCa2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MIAPaCa2 cells after 48 hrs by MTT assay, IC50=0.19μM. | 24041234 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50=0.2μM. | 24041234 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs by MTT assay, IC50=1.2μM. | 24041234 | ||
| MDA-MB-435 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435 cells after 72 hrs by MTT assay, IC50<0.001μM. | 24069881 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50=0.07μM. | 24069881 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50=0.65μM. | 24069881 | ||
| Hs683 | Growth inhibition assay | 72 hrs | Growth inhibition of human Hs683 cells after 72 hrs by MTT assay, IC50<0.01μM. | 24180210 | ||
| PC3 | Growth inhibition assay | 72 hrs | Growth inhibition of human PC3 cells after 72 hrs by MTT assay, IC50=0.01μM. | 24180210 | ||
| A549 | Growth inhibition assay | 72 hrs | Growth inhibition of human A549 cells after 72 hrs by MTT assay, IC50<0.01μM. | 24180210 | ||
| MCF7 | Growth inhibition assay | 72 hrs | Growth inhibition of human MCF7 cells after 72 hrs by MTT assay, IC50<0.01μM. | 24180210 | ||
| SK-MEL-28 | Growth inhibition assay | 72 hrs | Growth inhibition of human SK-MEL-28 cells after 72 hrs by MTT assay, IC50<0.01μM. | 24180210 | ||
| U373 | Growth inhibition assay | 72 hrs | Growth inhibition of human U373 cells after 72 hrs by MTT assay, IC50=0.015μM. | 24180210 | ||
| HT-29 | Antiproliferative assay | Antiproliferative activity against human HT-29 cells by SRB assay, IC50=0.0007μM. | 24200393 | |||
| MCF7 | Antiproliferative assay | Antiproliferative activity against human MCF7 cells by SRB assay, IC50=0.0033μM. | 24200393 | |||
| KB | Antiproliferative assay | Antiproliferative activity against human KB cells by SRB assay, IC50=0.02μM. | 24200393 | |||
| HeLa | Antiproliferative assay | Antiproliferative activity against human HeLa cells by SRB assay, IC50=0.13μM. | 24200393 | |||
| K562 | Cytotoxicity assay | 24 to 72 hrs | Cytotoxicity against human K562 cells after 24 to 72 hrs by MTT assay, IC50=0.4μM. | 24303808 | ||
| HeLa | Cytotoxicity assay | 24 to 72 hrs | Cytotoxicity against human HeLa cells after 24 to 72 hrs by MTT assay, IC50=0.5μM. | 24303808 | ||
| HepG2 | Cytotoxicity assay | 24 to 72 hrs | Cytotoxicity against human HepG2 cells after 24 to 72 hrs by MTT assay, IC50=0.6μM. | 24303808 | ||
| DU145 | Cytotoxicity assay | 24 to 72 hrs | Cytotoxicity against human DU145 cells after 24 to 72 hrs by MTT assay, IC50=0.8μM. | 24303808 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs by MTT assay, IC50=0.04μM. | 24360564 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50=0.05μM. | 24360564 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells after 48 hrs by MTT assay, IC50=0.06μM. | 24360564 | ||
| CCRF-CEM | Cytotoxicity assay | 24 hrs | Cytotoxicity against human CCRF-CEM cells assessed as cell viability after 24 hrs by MTT assay, IC50=0.08μM. | 24484281 | ||
| CEM/ADR5000 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human CEM/ADR5000 cells assessed as cell viability after 24 hrs by MTT assay, IC50=0.3μM. | 24484281 | ||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by SRB assay, GI50=0.01μM. | 24502276 | |||
| UACC62 | Cytotoxicity assay | Cytotoxicity against human UACC62 cells by SRB assay, GI50=0.01μM. | 24502276 | |||
| SF539 | Cytotoxicity assay | Cytotoxicity against human SF539 cells by SRB assay, GI50=0.01μM. | 24502276 | |||
| HOP62 | Cytotoxicity assay | Cytotoxicity against human HOP62 cells by SRB assay, GI50=0.01μM. | 24502276 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by SRB assay, GI50=0.013μM. | 24502276 | |||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, GI50=0.01738μM. | 24502276 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by MTT assay, GI50=0.0182μM. | 24502276 | ||
| SN12C | Cytotoxicity assay | Cytotoxicity against human SN12C cells by SRB assay, GI50=0.02μM. | 24502276 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells by SRB assay, GI50=0.03μM. | 24502276 | |||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells transfected with Top1-siRNA after 72 hrs by MTT assay, GI50=0.03548μM. | 24502276 | ||
| OVCAR3 | Cytotoxicity assay | Cytotoxicity against human OVCAR3 cells by SRB assay, GI50=0.22μM. | 24502276 | |||
| HCT116 | Function assay | 1 uM | 1 hr | Inhibition of human recombinant topoisomerase 1 in human HCT116 cells assessed as DNA-protein cross linking at 1 uM after 1 hr | 24502276 | |
| HCT116 | Function assay | 1 uM | 1 hr | Inhibition of human recombinant topoisomerase 1 in human HCT116 cells assessed as DNA-protein cross linking at 1 uM incubated for 1 hr followed by compound washout measured after 1 hr | 24502276 | |
| HCT116 | Function assay | 1 uM | 1 hr | Inhibition of human recombinant topoisomerase 1 in human HCT116 cells assessed as reversal of topoisomerase 1-DNA cleavage complex stabilization at 1 uM incubated for 1 hr followed by compound washout measured after 24 hrs by DNA immunoblotting analysis | 24502276 | |
| HCT116 | Function assay | 1 uM | 1 hr | Inhibition of human recombinant topoisomerase 1 in human HCT116 cells assessed as reversal of topoisomerase 1-DNA cleavage complex stabilization at 1 uM incubated for 1 hr followed by compound washout measured up to 6 hrs by DNA immunoblotting analysis | 24502276 | |
| HCT116 | Function assay | 1 uM | 1 hr | Inhibition of human recombinant topoisomerase 1 in human HCT116 cells assessed as stabilization of topoisomerase 1-DNA cleavage complex at 1 uM after 1 hr by DNA immunoblotting analysis | 24502276 | |
| B16F10 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse B16F10 cells after 48 hrs by MTT assay, IC50=2.78μM. | 24685545 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by SRB assay, GI50=0.01μM. | 24800942 | ||
| HOP62 | Growth inhibition assay | 48 hrs | Growth inhibition of human HOP62 cells after 48 hrs by SRB assay, GI50=0.01μM. | 24800942 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by SRB assay, GI50=0.01μM. | 24800942 | ||
| SF539 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF539 cells after 48 hrs by SRB assay, GI50=0.01μM. | 24800942 | ||
| UACC62 | Growth inhibition assay | 48 hrs | Growth inhibition of human UACC62 cells after 48 hrs by SRB assay, GI50=0.01μM. | 24800942 | ||
| SN12C | Growth inhibition assay | 48 hrs | Growth inhibition of human SN12C cells after 48 hrs by SRB assay, GI50=0.02μM. | 24800942 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT116 cells after 48 hrs by SRB assay, GI50=0.03μM. | 24800942 | ||
| OVCAR3 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR3 cells after 48 hrs by SRB assay, GI50=0.22μM. | 24800942 | ||
| MOLT4 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MOLT4 cells after 72 hrs by MTT assay, IC50=0.00004μM. | 24900725 | ||
| A2780 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A2780 cells after 72 hrs by MTT assay, IC50=0.013μM. | 24900725 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50=0.024μM. | 24900725 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50=0.026μM. | 24900725 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by MTT assay, IC50=0.031μM. | 24900725 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by MTT assay, IC50=0.078μM. | 24900725 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50=0.099μM. | 24900725 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50=0.162μM. | 24900725 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells overexpressing biotin receptor assessed as growth inhibition after 72 hrs by MTT assay, IC50=0.0651μM. | 24901491 | ||
| ID8 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse ID8 cells overexpressing biotin receptor assessed as growth inhibition after 72 hrs by MTT assay, IC50=0.474μM. | 24901491 | ||
| L1210 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse L1210 cells overexpressing folate receptor assessed as growth inhibition after 72 hrs by MTT assay, IC50=0.51μM. | 24901491 | ||
| WI38 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human WI38 cells overexpressing biotin receptor assessed as growth inhibition after 72 hrs by MTT assay, IC50=0.786μM. | 24901491 | ||
| MX1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MX1 cells overexpressing biotin receptor assessed as growth inhibition after 72 hrs by MTT assay, IC50=1.7μM. | 24901491 | ||
| MCF10A | Cytotoxicity assay | 2 days | Cytotoxicity against human MCF10A cells after 2 days, IC50=0.27μM. | 24904965 | ||
| T47D | Cytotoxicity assay | 2 days | Cytotoxicity against human T47D cells after 2 days, IC50=0.28μM. | 24904965 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days, IC50=4.01μM. | 24904965 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days, IC50=10.07μM. | 24904965 | ||
| Jurkat | Cytotoxicity assay | 24 hrs | Cytotoxicity against human Jurkat cells after 24 hrs by MTT assay, IC50=0.11μM. | 24980118 | ||
| Caco2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human Caco2 cells after 24 hrs by MTT assay, IC50=0.131μM. | 24980118 | ||
| A375 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A375 cells after 24 hrs by MTT assay, IC50=0.187μM. | 24980118 | ||
| MDA-MB-231 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MDA-MB-231 cells after 24 hrs by MTT assay, IC50=0.342μM. | 24980118 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTT assay, IC50=0.42μM. | 24980118 | ||
| MDA-MB-231 | Apoptosis assay | Induction of apoptosis in human MDA-MB-231 cells assessed as p53 phosphorylation at Ser15 by Western blotting analysis | 24980118 | |||
| HeLa | Apoptosis assay | 0.8 to 1.6 uM | 24 hrs | Induction of apoptosis in human HeLa cells assessed as inhibition of DNA synthesis at 0.8 to 1.6 uM after 24 hrs by BrdU staining-based ELISA | 24980118 | |
| MDA-MB-231 | Apoptosis assay | 0.8 to 1.6 uM | 24 hrs | Induction of apoptosis in human MDA-MB-231 cells assessed as inhibition of DNA synthesis at 0.8 to 1.6 uM after 24 hrs by BrdU staining-based ELISA | 24980118 | |
| A375 | Apoptosis assay | 0.8 to 1.6 uM | 24 hrs | Induction of apoptosis in human A375 cells assessed as inhibition of DNA synthesis at 0.8 to 1.6 uM after 24 hrs by BrdU staining-based ELISA | 24980118 | |
| A375 | Apoptosis assay | Induction of apoptosis in human A375 cells assessed as p53 phosphorylation at Ser15 by Western blotting analysis | 24980118 | |||
| Caco2 | Apoptosis assay | 0.8 to 1.6 uM | 24 hrs | Induction of apoptosis in human Caco2 cells assessed as inhibition of DNA synthesis at 0.8 to 1.6 uM after 24 hrs by BrdU staining-based ELISA | 24980118 | |
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, IC50=0.016μM. | 25003995 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by SRB assay, IC50=0.029μM. | 25003995 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by SRB assay, IC50=0.037μM. | 25003995 | ||
| KBVIN | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBVIN cells after 72 hrs by SRB assay, IC50=0.12μM. | 25003995 | ||
| A549 | Function assay | 30 to 100 nM | 30 mins | Inhibition of topoisomerase 1 in human A549 cells assessed as reduction in relaxation of supercoiled DNA substrate at 30 to 100 nM incubated for 30 mins by ethidium bromide staining based gel electrophoresis | 25003995 | |
| HEK293 | Cytotoxicity assay | 4 days | Cytotoxicity against HEK293 cells after 4 days by CCK-8 assay, IC50=0.28μM. | 25062006 | ||
| T47D | Cytotoxicity assay | 4 days | Cytotoxicity against human T47D cells after 4 days by CCK-8 assay, IC50=0.75μM. | 25062006 | ||
| DU145 | Cytotoxicity assay | 4 days | Cytotoxicity against human DU145 cells after 4 days by CCK-8 assay, IC50=0.95μM. | 25062006 | ||
| HCT15 | Cytotoxicity assay | 4 days | Cytotoxicity against human HCT15 cells after 4 days by CCK-8 assay, IC50=1.98μM. | 25062006 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by SRB assay, IC50=0.0005μM. | 25084144 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells in presence of 10 mM DTT after 48 hrs by SRB assay, IC50=0.003μM. | 25084144 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by SRB assay, IC50=1.3μM. | 25084144 | ||
| PC3 | Apoptosis assay | 2 uM | 12 hrs | Induction of apoptosis in human PC3 cells at 2 uM incubated for 12 hrs by DAPI staining based confocal laser microscopy | 25176329 | |
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50=0.001μM. | 25237727 | |||
| BGC823 | Cytotoxicity assay | Cytotoxicity against human BGC823 cells by MTT assay, IC50=0.04μM. | 25237727 | |||
| A2780 | Cytotoxicity assay | Cytotoxicity against human A2780 cells by MTT assay, IC50=0.9μM. | 25237727 | |||
| HCT8 | Cytotoxicity assay | Cytotoxicity against human HCT8 cells by MTT assay, IC50=3.6μM. | 25237727 | |||
| Bel7402 | Cytotoxicity assay | Cytotoxicity against human Bel7402 cells by MTT assay, IC50=6.3μM. | 25237727 | |||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by SRB assay, IC50=0.00015μM. | 25247770 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by SRB assay, IC50=0.0006μM. | 25247770 | ||
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells after 72 hrs by SRB assay, IC50=0.004μM. | 25247770 | ||
| SMMC7721 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SMMC7721 cells assessed as inhibition of cell viability after 48 hrs by MTT assay | 25453788 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as inhibition of cell viability after 48 hrs by MTT assay | 25453788 | ||
| MCF7 | Cytotoxicity assay | 2 days | Cytotoxicity against human MCF7 cells after 2 days by CCK-8 assay, IC50=0.08μM. | 25481396 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days by CCK-8 assay, IC50=0.11μM. | 25481396 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days by CCK-8 assay, IC50=0.26μM. | 25481396 | ||
| HeLa | Cytotoxicity assay | 2 days | Cytotoxicity against human HeLa cells after 2 days by CCK-8 assay, IC50=0.4μM. | 25481396 | ||
| K562 | Antitumor assay | 48 hrs | Antitumor activity against human K562 cells incubated for 48 hrs by MTT assay, IC50=0.13μM. | 25590864 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50=0.07μM. | 25599951 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs by MTT assay, IC50=5.37μM. | 25599951 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50=11.62μM. | 25599951 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50=26.45μM. | 25599951 | ||
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DU145 cells assessed as cell viability incubated for 72 hrs by MTT assay, IC50=0.1μM. | 25799376 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells assessed as cell viability incubated for 72 hrs by MTT assay, IC50=0.1μM. | 25799376 | ||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells measured on day 4 by CCK8 assay, IC50=0.02μM. | 25936262 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells measured on day 4 by CCK8 assay, IC50=0.18μM. | 25936262 | |||
| T47D | Cytotoxicity assay | Cytotoxicity against human T47D cells measured on day 4 by CCK8 assay, IC50=1.84μM. | 25936262 | |||
| HCT15 | Cytotoxicity assay | Cytotoxicity against human HCT15 cells measured on day 4 by CCK8 assay, IC50=2.82μM. | 25936262 | |||
| T47D | Cytotoxicity assay | 2 to 4 days | Cytotoxicity against human T47D cells incubated from day 2 to day 4 by CCK8 assay, IC50=0.31μM. | 26022080 | ||
| DU145 | Cytotoxicity assay | 2 to 4 days | Cytotoxicity against human DU145 cells incubated from day 2 to day 4 by CCK8 assay, IC50=0.4μM. | 26022080 | ||
| HeLa | Cytotoxicity assay | 2 to 4 days | Cytotoxicity against human HeLa cells incubated from day 2 to day 4 by CCK8 assay, IC50=0.41μM. | 26022080 | ||
| HCT15 | Cytotoxicity assay | 2 to 4 days | Cytotoxicity against human HCT15 cells incubated from day 2 to day 4 by CCK8 assay, IC50=0.52μM. | 26022080 | ||
| WI38 | Cytotoxicity assay | Cytotoxicity against human WI38 cells by MTT assay, IC50=0.23μM. | 26063305 | |||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50=0.015μM. | 26188908 | ||
| CA46 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CA46 cells after 48 hrs by MTT assay, IC50=0.52μM. | 26188908 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50=1.6μM. | 26188908 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50=14μM. | 26188908 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by MTT assay, IC50<0.003μM. | 26226379 | ||
| MV-3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MV-3 cells after 72 hrs by MTT assay, IC50<0.003μM. | 26226379 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50=0.09μM. | 26226379 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50=0.1μM. | 26226379 | ||
| Bel7404 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Bel7404 cells after 72 hrs by MTT assay, IC50=0.14μM. | 26226379 | ||
| SW579 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW579 cells after 72 hrs by MTT assay, IC50=0.71μM. | 26226379 | ||
| SKHEP1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKHEP1 cells after 72 hrs by MTT assay, IC50=0.96μM. | 26226379 | ||
| MDA-MB-435 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435 cells after 72 hrs by MTT assay, IC50=1μM. | 26226379 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by MTT assay, IC50=2.5μM. | 26226379 | ||
| Sf9 | Function assay | 10 uM | 5 mins | Inhibition of wild type recombinant human Top1 expressed in baculovirus infected insect Sf9 cells assessed as decrease in supercoiled pBS(SK+) DNA isomer relaxation at 10 uM preincubated for 5 mins followed by pBS(SK+) DNA addition by agarose gel electrop | 26312433 | |
| Sf9 | Function assay | 10 uM | 30 mins | Inhibition of wild type recombinant human Top1 expressed in baculovirus infected insect Sf9 cells assessed as decrease in supercoiled pBS(SK+) DNA isomer relaxation at 10 uM incubated simultaneously with enzyme and pBS(SK+) DNA for 30 mins by agarose gel | 26312433 | |
| Sf9 | Function assay | 50 uM | 30 mins | Inhibition of wild type recombinant human Top1 expressed in baculovirus infected insect Sf9 cells assessed as stabilization of Top1-cleavage complexes at 50 uM incubated for 30 mins by agarose gel electrophoresis | 26312433 | |
| HeLa | Cytotoxicity assay | 2 days | Cytotoxicity against human HeLa cells measured after 2 days by MTT assay, IC50=1.59μM. | 26334499 | ||
| T47D | Cytotoxicity assay | 2 days | Cytotoxicity against human T47D cells measured after 2 days by MTT assay, IC50=1.62μM. | 26334499 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells measured after 2 days by MTT assay, IC50=6.08μM. | 26334499 | ||
| T47D | Cytotoxicity assay | 2 days | Cytotoxicity against human T47D cells after 2 days by CCK8 assay, IC50=0.08μM. | 26361737 | ||
| DU145 | Cytotoxicity assay | 2 days | Cytotoxicity against human DU145 cells after 2 days by CCK8 assay, IC50=0.11μM. | 26361737 | ||
| HCT15 | Cytotoxicity assay | 2 days | Cytotoxicity against human HCT15 cells after 2 days by CCK8 assay, IC50=0.3μM. | 26361737 | ||
| MCF10A | Cytotoxicity assay | 2 days | Cytotoxicity against human MCF10A cells after 2 days by CCK8 assay, IC50=0.97μM. | 26361737 | ||
| 184B5 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human 184B5 cells after 72 hrs by SRB assay, IC50=4.96μM. | 26602827 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells after 72 hrs by SRB assay, IC50=5.77μM. | 26602827 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by SRB assay, IC50=6.13μM. | 26602827 | ||
| MDA-MB-468 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-468 cells after 72 hrs by SRB assay, IC50=14.76μM. | 26602827 | ||
| GM07492A | Cytotoxicity assay | 24 hrs | Cytotoxicity against human GM07492A cells assessed as cell growth inhibition after 24 hrs by XTT assay, IC50=3.2μM. | 26649907 | ||
| M059J | Cytotoxicity assay | 24 hrs | Cytotoxicity against human M059J cells assessed as cell growth inhibition after 24 hrs by XTT assay, IC50=5.5μM. | 26649907 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells assessed as cell growth inhibition after 24 hrs by XTT assay, IC50=6μM. | 26649907 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells assessed as cell growth inhibition after 24 hrs by XTT assay, IC50=19.8μM. | 26649907 | ||
| HT-29 | Function assay | 2 uM | 1 to 2 hrs | Induction of DNA damage in human HT-29 cells assessed as gamma-H2AX foci formation at 2 uM after 1 to 2 hrs by DAPI staining based indirect immunofluorescence assay | 26703795 | |
| HT-29 | Function assay | 2 uM | 1 to 2 hrs | Induction of DNA damage in human HT-29 cells assessed as increase in gamma-H2AX protein level at 2 uM after 1 to 2 hrs by Western blot analysis | 26703795 | |
| B16F10 | Cytotoxicity assay | 24 hrs | Cytotoxicity against mouse B16F10 cells overexpressing alphavbeta3 integrin up to 50 uM after 24 hrs by XTT assay | 26719208 | ||
| H1299 | Cytotoxicity assay | up to 50 uM | 24 hrs | Cytotoxicity against human H1299 cells overexpressing alphavbeta3 integrin at up to 50 uM after 24 hrs by XTT assay | 26719208 | |
| Mahlavu | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Mahlavu cells assessed as growth inhibition after 72 hrs by sulforhodamine B assay, IC50<1μM. | 26810835 | ||
| Hep3B | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Hep3B cells assessed as growth inhibition after 72 hrs by sulforhodamine B assay, IC50<1μM. | 26810835 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as growth inhibition after 72 hrs by sulforhodamine B assay, IC50<1μM. | 26810835 | ||
| SNU475 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SNU475 cells assessed as growth inhibition after 72 hrs by sulforhodamine B assay, IC50<1μM. | 26810835 | ||
| FOCUS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human FOCUS cells assessed as growth inhibition after 72 hrs by sulforhodamine B assay, IC50<1μM. | 26810835 | ||
| Jurkat | Anticancer assay | 48 hrs | Anticancer activity against human Jurkat cells after 48 hrs by MTT assay, IC50=0.085μM. | 26890120 | ||
| HeLa | Cytotoxicity assay | 3 days | Cytotoxicity against human HeLa cells after 3 days by CCK8 assay, IC50=0.59μM. | 26927425 | ||
| HCT15 | Cytotoxicity assay | 3 days | Cytotoxicity against human HCT15 cells after 3 days by CCK8 assay, IC50=1.29μM. | 26927425 | ||
| T47D | Cytotoxicity assay | 3 days | Cytotoxicity against human T47D cells after 3 days by CCK8 assay, IC50=11.17μM. | 26927425 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells after 48 hrs by CCK8 assay, IC50=0.02μM. | 26945111 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by CCK8 assay, IC50=0.18μM. | 26945111 | ||
| T47D | Cytotoxicity assay | 48 hrs | Cytotoxicity against human T47D cells after 48 hrs by CCK8 assay, IC50=1.84μM. | 26945111 | ||
| HCT15 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT15 cells after 48 hrs by CCK8 assay, IC50=2.82μM. | 26945111 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by CCK8 assay, IC50=4.1μM. | 26988802 | ||
| BT474 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human BT474 cells after 72 hrs by CCK8 assay, IC50=7μM. | 26988802 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells after 72 hrs by CCK8 assay, IC50=7.1μM. | 26988802 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by sulforhodamine B colorimetric assay, IC50=0.016μM. | 26994847 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by sulforhodamine B colorimetric assay, IC50=0.037μM. | 26994847 | ||
| KBVIN | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBVIN cells after 72 hrs by sulforhodamine B colorimetric assay, IC50=0.12μM. | 26994847 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition after 48 hrs by CCK8 assay, IC50=0.001μM. | 27017547 | ||
| SKOV3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKOV3 cells assessed as growth inhibition after 48 hrs by CCK8 assay, IC50=0.0055μM. | 27017547 | ||
| BT20 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human BT20 cells assessed as growth inhibition after 48 hrs by CCK8 assay, IC50=0.07μM. | 27017547 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs, GI50=0.01μM. | 27070999 | ||
| SF539 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SF539 cells after 48 hrs, GI50=0.01μM. | 27070999 | ||
| DU145 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human DU145 cells after 48 hrs, GI50=0.01μM. | 27070999 | ||
| HOP62 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HOP62 cells after 48 hrs, GI50=0.01μM. | 27070999 | ||
| UACC62 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human UACC62 cells after 48 hrs, GI50=0.01μM. | 27070999 | ||
| SN12C | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SN12C cells after 48 hrs, GI50=0.02μM. | 27070999 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs, GI50=0.03μM. | 27070999 | ||
| OVCAR3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human OVCAR3 cells after 48 hrs, GI50=0.22μM. | 27070999 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells assessed as reduction in cell viability after 72 hrs by ATPlite assay, IC50=0.003μM. | 27096049 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells assessed as reduction in cell viability after 72 hrs by ATPlite assay, IC50=0.009μM. | 27096049 | ||
| A375 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A375 cells assessed as reduction in cell viability after 72 hrs by ATPlite assay, IC50=0.013μM. | 27096049 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells assessed as reduction in cell viability after 72 hrs by ATPlite assay, IC50=0.035μM. | 27096049 | ||
| NCI-H23 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human NCI-H23 cells assessed as cell viability after 96 hrs by MTT assay, IC50=0.07μM. | 27156772 | ||
| NCI-H460 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human NCI-H460 cells assessed as cell viability after 96 hrs by MTT assay, IC50=0.19μM. | 27156772 | ||
| A549 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A549 cells assessed as cell viability after 96 hrs by MTT assay, IC50=0.35μM. | 27156772 | ||
| A549 | Function assay | 10 mM | 12 to 24 hrs | Drug uptake in human A549 cells at 10 mM after 12 to 24 hrs by flow cytometric method | 27156772 | |
| A549 | Function assay | 10 mM | 8 hrs | Drug uptake in human A549 cells at 10 mM after 8 hrs by flow cytometric method | 27156772 | |
| A549 | Growth inhibition assay | 72 hrs | Growth inhibition of human A549 cells after 72 hrs by MTT assay, IC50=0.12μM. | 27344492 | ||
| HepG2 | Growth inhibition assay | 72 hrs | Growth inhibition of human HepG2 cells after 72 hrs by MTT assay, IC50=29.81μM. | 27344492 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50=0.46μM. | 27416553 | ||
| BGC823 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human BGC823 cells after 48 hrs by MTT assay, IC50=1.27μM. | 27416553 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50=1.41μM. | 27416553 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50=1.52μM. | 27416553 | ||
| NCI60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI60 cells after 48 hrs by SRB assay, GI50=0.0398μM. | 27448924 | ||
| HCT15 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT15 cells assessed as reduction in cell growth after 72 hrs by CCK8 assay, IC50=0.0014μM. | 27484510 | ||
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells assessed as reduction in cell growth after 72 hrs by CCK8 assay, IC50=0.08μM. | 27484510 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells assessed as reduction in cell growth after 72 hrs by CCK8 assay, IC50=0.13μM. | 27484510 | ||
| NCI-N87 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-N87 cells assessed as reduction in cell growth after 72 hrs by CCK8 assay, IC50=0.6μM. | 27484510 | ||
| H460 | Growth inhibition assay | 72 hrs | Growth inhibition of human H460 cells after 72 hrs by trypan blue assay, GI50=0.002μM. | 27597409 | ||
| MSTO-211H | Growth inhibition assay | 72 hrs | Growth inhibition of human MSTO-211H cells after 72 hrs by trypan blue assay, GI50=0.0021μM. | 27597409 | ||
| HeLa | Growth inhibition assay | 72 hrs | Growth inhibition of human HeLa cells after 72 hrs by trypan blue assay, GI50=0.0054μM. | 27597409 | ||
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DU145 cells after 72 hrs by CCK8 assay, IC50=0.11μM. | 27643560 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells after 72 hrs by CCK8 assay, IC50=0.31μM. | 27643560 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by CCK8 assay, IC50=0.4μM. | 27643560 | ||
| HEK293 | Antiproliferative assay | 72 hrs | Antiproliferative activity against HEK293 cells after 72 hrs by CCK8 assay, IC50=0.83μM. | 27643560 | ||
| HFL1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against HFL1 cells after 72 hrs by CCK8 assay, IC50=1.06μM. | 27643560 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by CCK8 assay, IC50=13.7μM. | 27643560 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells measured after 72 hrs by CCK8 assay, IC50=0.35μM. | 27654394 | ||
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DU145 cells measured after 72 hrs by CCK8 assay, IC50=0.38μM. | 27654394 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells measured after 72 hrs by CCK8 assay, IC50=0.59μM. | 27654394 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells measured after 72 hrs by CCK8 assay, IC50=4.54μM. | 27654394 | ||
| ECA109 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human ECA109 cells assessed as decrease in cell proliferation after 48 hrs by MTT assay, IC50=9.52μM. | 27667553 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells assessed as decrease in cell proliferation after 48 hrs by MTT assay, IC50=11.36μM. | 27667553 | ||
| HEK293 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HEK293 cells measured after 72 hrs by MTT assay, IC50=0.89μM. | 27721148 | ||
| TE32 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human TE32 cells measured after 72 hrs by MTT assay, IC50=1.24μM. | 27721148 | ||
| MS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MS cells measured after 72 hrs by MTT assay, IC50=1.84μM. | 27721148 | ||
| Hep2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Hep2 cells measured after 72 hrs by MTT assay, IC50=3.04μM. | 27721148 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells measured after 72 hrs by MTT assay, IC50=5.93μM. | 27721148 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells measured after 72 hrs by MTT assay, IC50=6.7μM. | 27721148 | ||
| HCT116 | Apoptosis assay | 6.7 uM | 18 hrs | Induction of apoptosis in human HCT116 cells assessed as mitochondrial membrane permeability loss by measuring rhodamine 123 accumulation at 6.7 uM measured after 18 hrs by fluorescence inverted microscopic analysis | 27721148 | |
| HCT116 | Apoptosis assay | 6.7 uM | 18 hrs | Induction of apoptosis in human HCT116 cells assessed as chromatin condensation at 6.7 uM measured after 18 hrs by Hoechst 33258 staining based fluorescence inverted microscopic analysis | 27721148 | |
| HCT116 | Apoptosis assay | 6.7 uM | 18 hrs | Induction of apoptosis in human HCT116 cells assessed as appearance of half moon or crescent shape nuclei at 6.7 uM measured after 18 hrs by Hoechst 33258 staining based fluorescence inverted microscopic analysis | 27721148 | |
| HCT116 | Apoptosis assay | 6.7 uM | 18 hrs | Induction of apoptosis in human HCT116 cells assessed as nuclear fragmentation at 6.7 uM measured after 18 hrs by Hoechst 33258 staining based fluorescence inverted microscopic analysis | 27721148 | |
| HCT116 | Apoptosis assay | 6.7 uM | 18 hrs | Induction of apoptosis in human HCT116 cells assessed as formation of apoptotic bodies at 6.7 uM measured after 18 hrs by Hoechst 33258 staining based fluorescence inverted microscopic analysis | 27721148 | |
| A549 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human A549 cells after 96 hrs by MTT assay, IC50=0.006μM. | 27871039 | ||
| Bel7402 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human Bel7402 cells after 96 hrs by MTT assay, IC50=0.014μM. | 27871039 | ||
| HCT8 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human HCT8 cells after 96 hrs by MTT assay, IC50=0.024μM. | 27871039 | ||
| A2780 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human A2780 cells after 96 hrs by MTT assay, IC50=0.029μM. | 27871039 | ||
| BGC823 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human BGC823 cells after 96 hrs by MTT assay, IC50=0.057μM. | 27871039 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against vinblastine-sensitive human MCF7 cells assessed as growth inhibition after 72 hrs by SRB assay, IC50<0.0018μM. | 28006904 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against multidrug resistant human MCF7 cells cultured in vinblastine free medium assessed as growth inhibition after 72 hrs by SRB assay, IC50<0.0018μM. | 28006904 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against multidrug resistant human MCF7 cells cultured in vinblastine containing medium assessed as growth inhibition after 72 hrs by SRB assay, IC50<0.0018μM. | 28006904 | ||
| HEK293 | Cytotoxicity assay | 48 hrs | Cytotoxicity against HEK293 cells measured after 48 hrs by MTT assay, IC50=0.01μM. | 28027871 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50=0.24μM. | 28063351 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by CCK8 assay, IC50=0.09μM. | 28068603 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells after 72 hrs by CCK8 assay, IC50=0.6μM. | 28068603 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by CCK8 assay, IC50=2.1μM. | 28068603 | ||
| CCRF-CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CCRF-CEM cells assessed as reduction in cell viability after 72 hrs by resazurin reduction assay, IC50=0.003μM. | 28233677 | ||
| CEM/ADR5000 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CEM/ADR5000 cells assessed as reduction in cell viability after 72 hrs by resazurin reduction assay, IC50=0.011μM. | 28233677 | ||
| CCRF-CEM | Cell cycle assay | 0.01 uM | 6 hrs | Cell cycle arrest in human CCRF-CEM cells assessed as accumulation at S phase at 0.01 uM after 6 hrs by propidium iodide staining-based flow cytometry | 28233677 | |
| CCRF-CEM | Apoptosis assay | 0.01 uM | 72 hrs | Induction of apoptosis in human CCRF-CEM cells at 0.01 uM after 72 hrs by propidium iodide staining-based flow cytometry | 28233677 | |
| MCF7 | Antiproliferative assay | Antiproliferative activity against human MCF7 cells by SRB assay, IC50=0.03μM. | 28274626 | |||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by sulforhodamine B assay, IC50=0.00863μM. | 28285912 | ||
| KBVIN | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBVIN cells after 72 hrs by sulforhodamine B assay, IC50=0.0157μM. | 28285912 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by sulforhodamine B assay, IC50=0.05μM. | 28285912 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by sulforhodamine B assay, IC50=0.0875μM. | 28285912 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by sulforhodamine B assay, IC50=0.307μM. | 28285912 | ||
| Bel7402 | Function assay | 1 uM | 1 hr | Poison activity at DNA topoisomerase 1 in human Bel7402 cells assessed as induction of DNA damage by measuring upregulated gamma-H2AX levels at 1 uM after 1 hr by Western blot analysis | 28333459 | |
| Bel7402 | Function assay | 1 uM | 1 hr | Poison activity at DNA topoisomerase 1 in human Bel7402 cells assessed as induction of DNA damage by measuring upregulated levels of CHK1 phosphorylation at Ser345 residue at 1 uM after 1 hr by Western blot analysis | 28333459 | |
| Bel7402 | Function assay | 1 nM | 1 hr | Poison activity at DNA topoisomerase 1 in human Bel7402 cells assessed as induction of DNA damage by measuring upregulated levels of CHK2 phosphorylation at Thr68 residue at 1 nM after 1 hr by Western blot analysis | 28333459 | |
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50=0.12μM. | 28351590 | ||
| ZR-7530 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human ZR-7530 cells after 72 hrs by MTT assay, IC50=0.24μM. | 28351590 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50=0.26μM. | 28351590 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by CCK8 assay, IC50=0.06μM. | 28384547 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells after 72 hrs by CCK8 assay, IC50=0.26μM. | 28384547 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by CCK8 assay, IC50=2.1μM. | 28384547 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells after 3 days by sulforhodamine B assay, IC50=0.073μM. | 28409637 | ||
| HOP62 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HOP62 cells after 48 hrs by SRB assay, GI50<0.01μM. | 28418653 | ||
| SF539 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SF539 cells after 48 hrs by SRB assay, GI50<0.01μM. | 28418653 | ||
| UACC62 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human UACC62 cells after 48 hrs by SRB assay, GI50<0.01μM. | 28418653 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by SRB assay, GI50=0.013μM. | 28418653 | ||
| SN12C | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SN12C cells after 48 hrs by SRB assay, GI50=0.02μM. | 28418653 | ||
| OVCAR3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human OVCAR3 cells after 48 hrs by SRB assay, GI50=0.22μM. | 28418653 | ||
| CCRF-CEM | Function assay | 100 to 1000 nM | 2 hrs | Induction of DNA damage in human CCRF-CEM cells assessed as formation of gamma-H2AX foci at 100 to 1000 nM after 2 hrs by DAPI staining-based confocal microscopy | 28418653 | |
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells assessed as reduction in cell viability by MTT assay, IC50=0.12μM. | 28425292 | |||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50=0.0115μM. | 28454671 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells after 48 hrs by MTT assay, IC50=9.45μM. | 28462840 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50=10.1μM. | 28462840 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50=13.64μM. | 28462840 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50=28.66μM. | 28462840 | ||
| HL60 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HL60 cells measured after 48 hrs by WST1 assay, IC50=0.019μM. | 28477443 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells measured after 48 hrs by MTT assay, IC50=0.159μM. | 28477443 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50=0.7μM. | 28506750 | |||
| NCI-H1299 | Cytotoxicity assay | Cytotoxicity against human NCI-H1299 cells by MTT assay, IC50=0.8μM. | 28506750 | |||
| NCI-H1975 | Cytotoxicity assay | Cytotoxicity against human NCI-H1975 cells by MTT assay, IC50=1.2μM. | 28506750 | |||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50<1μM. | 28511910 | ||
| QSG7701 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human QSG7701 cells after 48 hrs by MTT assay, IC50<1μM. | 28511910 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50=3.8μM. | 28511910 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50=4.92μM. | 28511910 | ||
| WI38 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human WI38 cells after 48 hrs by WST-1 assay, IC50=0.03μM. | 28557449 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by WST-1 assay, IC50=0.08μM. | 28557449 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells after 72 hrs by CCK8 assay, IC50=0.76μM. | 28633898 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by CCK8 assay, IC50=2.31μM. | 28633898 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by CCK8 assay, IC50=2.62μM. | 28633898 | ||
| HOP62 | Antiproliferative assay | Antiproliferative activity against human HOP62 cells assessed as growth inhibition, GI50=0.01μM. | 28657311 | |||
| SF539 | Antiproliferative assay | Antiproliferative activity against human SF539 cells assessed as growth inhibition, GI50=0.01μM. | 28657311 | |||
| UACC62 | Antiproliferative assay | Antiproliferative activity against human UACC62 cells assessed as growth inhibition, GI50=0.01μM. | 28657311 | |||
| DU145 | Antiproliferative assay | Antiproliferative activity against human DU145 cells assessed as growth inhibition, GI50=0.01μM. | 28657311 | |||
| MCF7 | Antiproliferative assay | Antiproliferative activity against human MCF7 cells assessed as growth inhibition, GI50=0.013μM. | 28657311 | |||
| SN12C | Antiproliferative assay | Antiproliferative activity against human SN12C cells assessed as growth inhibition, GI50=0.02μM. | 28657311 | |||
| HCT116 | Antiproliferative assay | Antiproliferative activity against human HCT116 cells assessed as growth inhibition, GI50=0.03μM. | 28657311 | |||
| OVCAR3 | Antiproliferative assay | Antiproliferative activity against human OVCAR3 cells assessed as growth inhibition, GI50=0.22μM. | 28657311 | |||
| CCRF-CEM | Function assay | 0.1 to 1 uM | 2 hrs | Induction of DNA damage in human CCRF-CEM cells assessed as gamma-H2AX foci formation at 0.1 to 1 uM after 2 hrs by confocal microscopy | 28657311 | |
| MIAPaCa2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MIAPaCa2 cells after 48 hrs in presence of type 1 collagen coated surface by MTT assay, IC50=0.18μM. | 28689976 | ||
| PC3 | Growth inhibition assay | 24 hrs | Growth inhibition of human PC3 cells up to 25 uM cotreated with colchicine measured after 24 hrs by XTT assay | 28710962 | ||
| MDA-MB-231 | Growth inhibition assay | 24 hrs | Growth inhibition of human MDA-MB-231 cells up to 25 uM cotreated with colchicine measured after 24 hrs by XTT assay | 28710962 | ||
| WM266.4 | Growth inhibition assay | 24 hrs | Growth inhibition of human WM266.4 cells up to 25 uM cotreated with colchicine measured after 24 hrs by XTT assay | 28710962 | ||
| A172 | Growth inhibition assay | 24 hrs | Growth inhibition of human A172 cells up to 25 uM cotreated with colchicine measured after 24 hrs by XTT assay | 28710962 | ||
| NCI-H1299 | Growth inhibition assay | 24 hrs | Growth inhibition of human NCI-H1299 cells up to 25 uM cotreated with colchicine measured after 24 hrs by XTT assay | 28710962 | ||
| insect cells | Function assay | 80 uM | 30 mins | Inhibition of human topoisomerase-1 expressed in baculovirus infected insect cells at 80 uM using supercoiled pGEM-5Zf(+) plasmid DNA as substrate after 30 mins by agarose gel electrophoresis method | 28753517 | |
| RD | Cytotoxicity assay | 72 hrs | Cytotoxicity in human RD cells assessed as inhibition of cell growth incubated for 72 hrs by MTT assay, IC50=4.49μM. | 28923382 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity in human A549 cells assessed as inhibition of cell growth incubated for 72 hrs by MTT assay, IC50=8.87μM. | 28923382 | ||
| HCT16 | Cytotoxicity assay | 72 hrs | Cytotoxicity in human HCT16 cells assessed as inhibition of cell growth incubated for 72 hrs by MTT assay, IC50=12.34μM. | 28923382 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity in human MCF7 cells assessed as inhibition of cell growth incubated for 72 hrs by MTT assay, IC50=13.62μM. | 28923382 | ||
| TE32 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human TE32 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50=1.24μM. | 28923388 | ||
| MS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MS cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50=1.84μM. | 28923388 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50=1.88μM. | 28923388 | ||
| Hep2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Hep2 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50=3.04μM. | 28923388 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50=5.93μM. | 28923388 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, IC50=0.00863μM. | 28927790 | ||
| KBVIN | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBVIN cells after 72 hrs by SRB assay, IC50=0.0157μM. | 28927790 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by SRB assay, IC50=0.05μM. | 28927790 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by SRB assay, IC50=0.0875μM. | 28927790 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by SRB assay, IC50=0.307μM. | 28927790 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by tryphan blue staining based hematocytometric method, IC50=0.92μM. | 29131616 | ||
| PC3 | Antiproliferative assay | Antiproliferative activity against human PC3 cells by MTT assay, IC50=6.96μM. | 29131616 | |||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells assessed as reduction in cell viability after 48 hrs by ATPlite-based luminescent assay, IC50=0.02μM. | 29223099 | ||
| HL60 | Apoptosis assay | 24 hrs | Induction of apoptosis in human HL60 cells assessed as caspase 3/7 activity after 24 hrs by caspase-glo luminescent assay, IC50=0.07μM. | 29223099 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells assessed as reduction in cell viability after 48 hrs by ATPlite-based luminescent assay, IC50=0.09μM. | 29223099 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as reduction in cell viability after 48 hrs by ATPlite-based luminescent assay, IC50=0.34μM. | 29223099 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as reduction in cell viability after 48 hrs by ATPlite-based luminescent assay, IC50=0.4μM. | 29223099 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 48 hrs by ATPlite-based luminescent assay, IC50=0.43μM. | 29223099 | ||
| K562 | Apoptosis assay | 24 hrs | Induction of apoptosis in human K562 cells assessed as caspase 3/7 activity after 24 hrs by caspase-glo luminescent assay, IC50=0.711μM. | 29223099 | ||
| HepG2 | Apoptosis assay | 24 hrs | Induction of apoptosis in human HepG2 cells assessed as caspase 3/7 activity after 24 hrs by caspase-glo luminescent assay, IC50=4.37μM. | 29223099 | ||
| RPE1 | Apoptosis assay | 24 hrs | Induction of apoptosis in human RPE1 cells assessed as caspase 3/7 activity after 24 hrs by caspase-glo luminescent assay, IC50=27.2μM. | 29223099 | ||
| Sf9 | Function assay | 10 to 50 uM | 30 mins | Stabilization of recombinant human topoisomerase-1-supercoiled pBS SK(+) DNA cleavage complex at 10 to 50 uM after 30 mins using topoisomerase1 expressed in baculovirus infected cells by ethidium bromide staining based agarose gel electrophoresis meth | 29290109 | |
| MCF7 | Function assay | 10 uM | 12 hrs | Stabilization of topoisomerase-1- genomic DNA cleavage complex in human MCF7 cells at 10 uM after 12 hrs by ICE assay | 29290109 | |
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells incubated for 72 hrs by CCK8 assay, IC50=0.05μM. | 29402741 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells incubated for 72 hrs by CCK8 assay, IC50=0.07μM. | 29402741 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells incubated for 72 hrs by CCK8 assay, IC50=1.37μM. | 29402741 | ||
| HeLa | Antiproliferative assay | 24 hrs | Antiproliferative activity against human HeLa cells after 24 hrs by crystal violet staining-based assay, IC50=0.94μM. | 29407986 | ||
| CaSki | Antiproliferative assay | 24 hrs | Antiproliferative activity against human CaSki cells after 24 hrs by crystal violet staining-based assay, IC50=1.8μM. | 29407986 | ||
| ViBo | Antiproliferative assay | 24 hrs | Antiproliferative activity against human ViBo cells after 24 hrs by crystal violet staining-based assay, IC50=1.92μM. | 29407986 | ||
| HL60 | Antiproliferative assay | Antiproliferative activity against human HL60 cells, IC50=0.0067μM. | 29429833 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB1643 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-12 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for LAN-5 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for DAOY cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB-EBc1 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SJ-GBM2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for RD cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh18 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for OHS-50 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-SH cells | 29435139 | |||
| HEK293 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HEK293 cells after 48 hrs by CCK-8 assay, IC50=0.00056μM. | 29501943 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by CCK-8 assay, IC50=0.001μM. | 29501943 | ||
| SKOV3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SKOV3 cells after 48 hrs by CCK-8 assay, IC50=0.0055μM. | 29501943 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells after 72 hrs by CCK-8 assay, IC50=0.02μM. | 29510948 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by CCK-8 assay, IC50=0.13μM. | 29510948 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by CCK-8 assay, IC50=0.49μM. | 29510948 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=0.11μM. | 29541373 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=0.16μM. | 29541373 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=0.54μM. | 29541373 | ||
| CCRF-CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CCRF-CEM cells assessed as growth inhibition after 72 hrs by MTT assay, GI50=0.002μM. | 29705710 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition after 72 hrs by MTT assay, GI50=0.003μM. | 29705710 | ||
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells assessed as growth inhibition after 72 hrs by MTT assay, GI50=0.006μM. | 29705710 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells assessed as growth inhibition after 72 hrs by MTT assay, GI50=0.009μM. | 29705710 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells assessed as growth inhibition after 72 hrs by MTT assay, GI50=0.019μM. | 29705710 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells expressing TOP1 siRNA assessed as growth inhibition after 72 hrs by MTT assay, GI50=0.075μM. | 29705710 | ||
| RC0.1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human RC0.1 cells assessed as growth inhibition after 72 hrs by MTT assay, GI50=7.53μM. | 29705710 | ||
| THP1 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human THP1 cells after 24 hrs by LDH release assay, LD50=0.161μM. | 29773506 | ||
| A549 | Function assay | 2 uM | 2 hrs | Induction of DNA damage in human A549 cells assessed as gamma-H2AX foci formation at 2 uM after 2 hrs by DAPI staining based immunofluorescence assay | 29843103 | |
| A549 | Function assay | 2 uM | 2 hrs | Induction of DNA damage in human A549 cells assessed as increase in gamma-H2AX level at 2 uM after 2 hrs by immunoblot analysis | 29843103 | |
| A549 | Function assay | 2 uM | 2 hrs | Induction of DNA damage in human A549 cells assessed as increase in CHK1 phosphorylation level at 2 uM after 2 hrs by immunoblot analysis | 29843103 | |
| HepG2 | Antiproliferative assay | Antiproliferative activity against human HepG2 cells by MTT assay, IC50=0.5μM. | 30007563 | |||
| HeLa | Antiproliferative assay | Antiproliferative activity against human HeLa cells by MTT assay, IC50=1.3μM. | 30007563 | |||
| PANC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PANC1 cells after 72 hrs by XTT assay, IC50=0.09μM. | 30017114 | ||
| PANC1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PANC1 cells after 48 hrs by XTT assay, IC50=0.18μM. | 30017114 | ||
| Capan1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Capan1 cells after 72 hrs by XTT assay, IC50=0.21μM. | 30017114 | ||
| CNE2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CNE2 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50=0.1μM. | 30025344 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50=5.5μM. | 30025344 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50=13.2μM. | 30025344 | ||
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, IC50=0.07μM. | 30055465 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50=0.37μM. | 30055465 | ||
| CNE1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CNE1 cells after 72 hrs by MTT assay, IC50=0.34μM. | 30125725 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50=0.42μM. | 30125725 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50=0.66μM. | 30125725 | ||
| CNE2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CNE2 cells after 72 hrs by MTT assay, IC50=0.98μM. | 30125725 | ||
| SMMC7721 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SMMC7721 cells after 72 hrs by MTT assay, IC50=1.08μM. | 30125725 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50=1.36μM. | 30125725 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50=6.08μM. | 30125725 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells after 72 hrs by EZ-CYTOX reagent based assay, IC50=0.05μM. | 30262132 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by EZ-CYTOX reagent based assay, IC50=0.07μM. | 30262132 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by EZ-CYTOX reagent based assay, IC50=1.37μM. | 30262132 | ||
| CCRF-CEM | Growth inhibition assay | 72 hrs | Growth inhibition of human CCRF-CEM cells after 72 hrs by MTT assay, GI50=0.007μM. | 30336023 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells after 72 hrs by MTT assay, GI50=0.009μM. | 30336023 | ||
| DU145 | Growth inhibition assay | 72 hrs | Growth inhibition of human DU145 cells after 72 hrs by MTT assay, GI50=0.021μM. | 30336023 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells expressing TOP1 siRNA after 72 hrs by MTT assay, GI50=0.075μM. | 30336023 | ||
| HuH7 | Growth inhibition assay | 72 hrs | Growth inhibition of human HuH7 cells after 72 hrs by MTT assay, GI50=0.099μM. | 30336023 | ||
| A549 | Growth inhibition assay | 72 hrs | Growth inhibition of human A549 cells after 72 hrs by MTT assay, GI50=0.21μM. | 30336023 | ||
| RC0.1 | Growth inhibition assay | 72 hrs | Growth inhibition of human RC0.1 cells after 72 hrs by MTT assay, GI50=4.73μM. | 30336023 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against against human MCF7 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=0.04μM. | 30340899 | ||
| MS | Cytotoxicity assay | 72 hrs | Cytotoxicity against against human MS cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=0.77μM. | 30340899 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against against human A549 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=1.31μM. | 30340899 | ||
| HEK293 | Cytotoxicity assay | 72 hrs | Cytotoxicity against HEK293 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=1.61μM. | 30340899 | ||
| RD | Cytotoxicity assay | 72 hrs | Cytotoxicity against against human RD cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=1.72μM. | 30340899 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against against human HCT116 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=1.88μM. | 30340899 | ||
| Hep2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against against human Hep2 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50=3.01μM. | 30340899 | ||
| HCT116 | Antimigratory assay | 2 uM | 96 hrs | Antimigratory activity in human HCT116 cells at 2 uM after 96 hrs by wound healing assay | 30340899 | |
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50<0.064μM. | 30509782 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50=0.09μM. | 30509782 | ||
| SW480 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SW480 cells after 48 hrs by MTT assay, IC50=0.29μM. | 30509782 | ||
| SMMC7721 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SMMC7721 cells after 48 hrs by MTT assay, IC50=0.45μM. | 30509782 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50=0.68μM. | 30509782 | ||
| CCRF-CEM | Cytotoxicity assay | Cytotoxicity against human CCRF-CEM cells by MTT assay, IC50=0.002μM. | 30543429 | |||
| HuH7 | Cytotoxicity assay | Cytotoxicity against human HuH7 cells by MTT assay, IC50=0.0023μM. | 30543429 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells by MTT assay, IC50=0.013μM. | 30543429 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by MTT assay, IC50=0.016μM. | 30543429 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50=0.04μM. | 30543429 | |||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=0.66μM. | 30600208 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50=0.68μM. | 30600208 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells after 48 hrs by MTT assay, IC50=2.96μM. | 30685528 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50=4.43μM. | 30685528 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50=4.79μM. | 30685528 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50=6.51μM. | 30685528 | ||
| T24 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T24 cells after 48 hrs by MTT assay, IC50=6.8μM. | 30685528 | ||
| WI38 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human WI38 cells after 48 hrs by MTT assay, IC50=8.37μM. | 30685528 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by CCK8 assay, IC50=0.00032μM. | 30702286 | ||
| LN229 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human LN229 cells after 48 hrs by CCK8 assay, IC50=0.00032μM. | 30702286 | ||
| MHCC97H | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MHCC97H cells after 48 hrs by CCK8 assay, IC50=0.00032μM. | 30702286 | ||
| LoVo | Cytotoxicity assay | 48 hrs | Cytotoxicity against human LoVo cells after 48 hrs by CCK8 assay, IC50=0.00032μM. | 30702286 | ||
| MDA231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA231 cells after 48 hrs by CCK8 assay, IC50=0.00032μM. | 30702286 | ||
| HuH7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HuH7 cells after 48 hrs by CCK8 assay, IC50=0.00032μM. | 30702286 | ||
| MGC | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC cells after 48 hrs by CCK8 assay, IC50=0.00032μM. | 30702286 | ||
| HuH7 | Apoptosis assay | Induction of apoptosis in human HuH7 cells assessed as increase in cytochrome C levels by Hoechst staining-based assay | 30716712 | |||
| Mahlavu | Apoptosis assay | Induction of apoptosis in human Mahlavu cells assessed as increase in cytochrome C levels by Hoechst staining-based assay | 30716712 | |||
| HL7702 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL7702 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50=2.15μM. | 30875505 | ||
| T24 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T24 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50=3.09μM. | 30875505 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50=4.27μM. | 30875505 | ||
| NCI-H460 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human NCI-H460 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50=5.37μM. | 30875505 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50=5.69μM. | 30875505 | ||
| Sf9 | Function assay | 30 mins | Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA mobility measured after 30 mins by ethidium bromide staining based agarose gel electrophoresis, IC50=0.025μM. | 30897325 | ||
| MCF7 | Function assay | 30 mins | Poison activity at TOP1 in human MCF7 cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA mobility measured after 30 mins by ethidium bromide staining based agarose gel electrophoresis, IC50=2.5μM. | 30897325 | ||
| Sf9 | Function assay | 10 to 25 uM | 30 mins | Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as stabilization of enzyme-pBS(SK+) DNA cleavable complex formation at 10 to 25 uM measured after 30 mins by ethidium bromide staining based agarose gel | 30897325 | |
| Sf9 | Function assay | 5 to 25 uM | 30 mins | Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as stabilization of enzyme-25mer duplex oligonucleotide cleavable complex formation at 5 to 25 uM measured after 30 mins by ethidium bromide staining bas | 30897325 | |
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability measured after 72 hrs by MTT assay, IC50=3.32μM. | 30902399 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells incubated for 72 hrs by MTT assay, GI50=0.009μM. | 31176097 | ||
| MCF7 | Growth inhibition assay | 72 hrs | Growth inhibition of human MCF7 cells incubated for 72 hrs by MTT assay, GI50=0.012μM. | 31176097 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells expressing siTOP1 incubated for 72 hrs by MTT assay, GI50=0.075μM. | 31176097 | ||
| A549 | Growth inhibition assay | 72 hrs | Growth inhibition of human A549 cells incubated for 72 hrs by MTT assay, GI50=0.099μM. | 31176097 | ||
| DU145 | Growth inhibition assay | 72 hrs | Growth inhibition of human DU145 cells incubated for 72 hrs by MTT assay, GI50=0.21μM. | 31176097 | ||
| DU145/RC0.1 | Growth inhibition assay | 72 hrs | Growth inhibition of human DU145/RC0.1 cells harboring TOP1 R364H mutant incubated for 72 hrs by MTT assay, GI50=4.73μM. | 31176097 | ||
| HCT116 | Function assay | 25 uM | 1 hr | Inhibition of TOP1 in human HCT116 cells assessed as induction of cellular TOP1cc formation at 25 uM incubated for 1 hr by chemiluminescence based assay | 31176097 | |
| HCT116 | Function assay | 1 uM | 3 hrs | Inhibition of TOP1 in human HCT116 cells assessed as induction of DNA damage by measuring increase in gammaH2AX foci at 1 uM incubated for 3 hrs by DAPI staining based immunofluorescence microscopic method | 31176097 | |
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells incubated for 48 hrs by CCK8 assay, IC50=0.001μM. | 31229877 | ||
| SKOV3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SKOV3 cells incubated for 48 hrs by CCK8 assay, IC50=0.0055μM. | 31229877 | ||
| HCC70 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCC70 cells measured after 72 hrs by Ez-cytox assay, IC50=0.044μM. | 31398033 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells measured after 72 hrs by EZ-Cytox assay, IC50=0.049μM. | 31398033 | ||
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DU145 cells measured after 72 hrs by EZ-Cytox assay, IC50=0.05μM. | 31398033 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells measured after 72 hrs by EZ-Cytox assay, IC50=0.25μM. | 31398033 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells measured after 72 hrs by EZ-Cytox assay, IC50=0.5μM. | 31398033 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells measured after 72 hrs by Ez-cytox assay, IC50=23.98μM. | 31398033 | ||
| MDA-MB-436 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-436 cells measured after 72 hrs by Ez-cytox assay, IC50=31.86μM. | 31398033 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells assessed as reduction in cell viability after 48 hrs by SRB assay, IC50=0.03μM. | 31546197 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells assessed as reduction in cell viability after 48 hrs by SRB assay, IC50=0.03μM. | 31546197 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells assessed as reduction in cell viability after 48 hrs by SRB assay, IC50=0.03μM. | 31546197 | ||
| HT-29 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HT-29 cells assessed as reduction in cell viability after 48 hrs by SRB assay, IC50=0.03μM. | 31546197 | ||
| MV-3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MV-3 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=0.003μM. | 31880942 | ||
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50<0.003μM. | 31880942 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=0.02μM. | 31880942 | ||
| Bel7404 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7404 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=0.4μM. | 31880942 | ||
| SW579 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW579 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=0.7μM. | 31880942 | ||
| SKHEP1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SKHEP1 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=0.9μM. | 31880942 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=2.4μM. | 31880942 | ||
| MDA-MB-435 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-435 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=2.7μM. | 31880942 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells assessed as reduction in cell viability incubated for 72 hrs by CCK8 assay, IC50=2.8μM. | 31880942 | ||
| HL60 | Function assay | Tested in vitro for growth inhibitory activity against HL60 (human promyelocytic leukemic) cells, IC50=0.005μM. | ChEMBL | |||
| DC-3F | Function assay | Tested in vitro for growth inhibitory activity against DC-3F (hamster lung) cells, IC50=0.006μM. | ChEMBL | |||
| HCT116 | Antiproliferative assay | 4 days | Antiproliferative activity against human HCT116 cells assessed as reduction in cell viability after 4 days by [3H]-thymidine incorporation assay, IC50=0.0085μM. | ChEMBL | ||
| 833K | Function assay | Tested in vitro for growth inhibitory activity against 833K (human teratorcarcinoma) cells, IC50=0.01μM. | ChEMBL | |||
| 786-O | Antiproliferative assay | 72 hrs | Antiproliferative activity against human 786-O cells incubated for 72 hrs by MTT assay, IC50=0.015μM. | ChEMBL | ||
| MDA-MB-231 | Antiproliferative assay | 4 days | Antiproliferative activity against human MDA-MB-231 cells assessed as reduction in cell viability after 4 days by [3H]-thymidine incorporation assay, IC50=0.0284μM. | ChEMBL | ||
| HuH7 | Cytotoxicity assay | Cytotoxicity against human HuH7 cells assessed as inhibition of cell proliferation by sulforhodamine B assay, IC50=0.1μM. | ChEMBL | |||
| leukemia cells | Function assay | Compound was evaluated for cytotoxic activity on L1210 murine leukemia cells, IC50=0.126μM. | ChEMBL | |||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells incubated for 72 hrs by MTT assay, IC50=0.18μM. | ChEMBL | ||
| MIAPaCa2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MIAPaCa2 cells assessed as cell viability after 48 hrs by MTT assay, IC50=0.19μM. | ChEMBL | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells assessed as cell viability after 48 hrs by MTT assay, IC50=0.2μM. | ChEMBL | ||
| SKHep | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SKHep cells incubated for 72 hrs by MTT assay, IC50=0.22μM. | ChEMBL | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50=0.42μM. | ChEMBL | ||
| Detroit 551 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Detroit 551 cells incubated for 72 hrs by MTT assay, IC50=0.99μM. | ChEMBL | ||
| MV | Cytotoxicity assay | Cytotoxicity against human MV cells assessed as inhibition of cell proliferation by sulforhodamine B assay, IC50<1μM. | ChEMBL | |||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells assessed as cell viability after 48 hrs by MTT assay, IC50=1.2μM. | ChEMBL | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells incubated for 72 hrs by MTT assay, IC50=2.11μM. | ChEMBL | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50=2.21μM. | ChEMBL | ||
| H1299 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human H1299 cells incubated for 72 hrs by MTT assay, IC50=3.14μM. | ChEMBL | ||
| Raji | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Raji cells after 48 hrs by MTT assay, IC50=3.27μM. | ChEMBL | ||
| SW480 | Function assay | Inhibition of DNA topoisomerase 1 in Homo sapiens (human) SW480 cells, IC50=3.75μM. | ChEMBL | |||
| HCT116 | Function assay | Inhibition of DNA topoisomerase 1 in Homo sapiens (human) HCT116 cells, IC50=4.75μM. | ChEMBL | |||
| VACO 241 | Function assay | Inhibition of DNA topoisomerase 1 in Homo sapiens (human) VACO 241 cells, IC50=4.95μM. | ChEMBL | |||
| SAS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SAS cells incubated for 72 hrs by MTT assay, IC50=6μM. | ChEMBL | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50=16.44μM. | ChEMBL | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs by MTT assay, IC50=18.24μM. | ChEMBL | ||
| AGS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human AGS cells incubated for 72 hrs by MTT assay, IC50=23.76μM. | ChEMBL | ||
| MCF7 | Function assay | 10 uM | 12 hrs | Poisoning activity at topoisomerase 1 in human MCF7 cells at 10 uM after 12 hrs by immunoband depletion assay | ChEMBL | |
| A549 | Apoptosis assay | 12 to 15 hrs | Induction of apoptosis in human A549 cells assessed as formation of apoptotic bodies after 12 to 15 hrs by flow cytometric method | ChEMBL | ||
| A549 | Apoptosis assay | 12 to 15 hrs | Induction of apoptosis in human A549 cells assessed as cell condensation after 12 to 15 hrs by flow cytometric method | ChEMBL | ||
| MDA-MB-231 | Cell cycle assay | 1 uM | 16 hrs | Cell cycle arrest in human MDA-MB-231 cells assessed as accumulation at S-phase at 1 uM incubated for 16 hrs by flow cytometry | ChEMBL | |
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Description | Camptothecin (CPT) is a specific inhibitor of DNA topoisomerase I (Topo I) with IC50 of 0.68 μM in a cell-free assay. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Phase 2. | ||
|---|---|---|---|
| Targets |
|
In vitro |
||||
| In vitro | Camptothecin, a plant alkaloid orignially isolated from Camptotheca acuminate in 1966. [1] Camptothecin is noted to halt cells during the S phase of mitosis. Camptothecin displays nanomolar potency in cytotoxicity against many human tumor cell lines, including HT29, LOX, SKOV3, and SKVLB, with IC50 values ranging from 37 nM to 48 nM. [2] In combination with TNF, Camptothecin induces apoptosis in primary mouse hepatocytes, with an IC50 value of 13 μM. Camptothecin also abrogated the TNF-induced NF-κB Activation, as well as the expression of TNF-receptor associated factor 2 (TRAF2), X-linked inhibitor of apoptosis protein (X-IAP), and FLICE-inhibitory protein (FLIP). [4] In HCT116 cells, Camptothecin (5 μM) induces proteasome-mediated degradation of mixed lineage leukemia 5 (MLL5) protein, which leads to phosphorylation of p53 at Ser392. [5] Due to the low solubility and adverse effects of Camptothecin, various Camptothecin analogues have been developed, and two of them, topotecan and irinotecan, has been approved by FDA and are used in cancer chemotherapy. | |||
|---|---|---|---|---|
| Kinase Assay | Topoisomerase I Cleavable Complex Assay | |||
| Topoisomerase I is isolated from calf thymus and is devoid of topoisomerase II. All reactions are carried out in 10 mL volumes of reaction buffer (50 mM Tris-HCl, pH 7.5, 100 mM KCl, 0.5 mM EDTA, and 30 pg/mL BSA) in microtiter plates. Camptothecin is dissolved in DMSO at 10 mg/mL and serially diluted in 96-well microtiter plates to which the 32P end-labeled pBR322 DNA and topoisomerase enzyme are added. The reaction mixture is incubated at room temperature for 30 min and then the reaction stopped by adding 2 mL of a mixture of sodium dodecyl sulfate and proteinase K (1.6% and 0.14 mg/mL final concentrations, respectively). The plates are heated at 50 °C for 30 min, 10 mL of standard stop mixture containing 0.45 N NaOH is added in order to generate single-stranded DNA, and the samples are electrophoresed in 1.5% agarose gels in TBE buffer. Gels are blotted on nitrocellulose paper, dried, and exposed to X-ray film. The units of cleavage are calculated from the autoradiographs and plotted against the log drug concentration. The IC50 values are then obtaine | ||||
| Cell Research | Cell lines | U87MG, A549 and H838 cells | ||
| Concentrations | 0.17 nM–10 mM | |||
| Incubation Time | 48 hours | |||
| Method | Tumor cells are plated in 100 μL of medium in 96-well microtiter plates at a density of 1500 to 4000 cells per well and allowed to adhere overnight. Cells are incubated with Camptothecin for 48 hours and then with fresh medium for 48 hours. Camptothecin at each concentration is added in quadruplicate. Following a 4-hour incubation of treated cells with MTT, the reduced dye product is extracted from the cells with 0.2 mL of DMSO followed by 50 μL of Sorensen's buffer. The plates are shaken briefly, and the absorbance at 570 nm is read and quantitated. Curves are fitted to the MTT assay data using a four-parameter logistic equation. | |||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | Cyclin1 / CDK1 / CDK2 Vimentin / Fibronectin / E-cadherin TGF-β / PI3K / pPI3K / AKT / pAKT p53 / Cleaved caspase-3 / Cleaved PARP |
|
29963130 | |
In Vivo |
||
| In vivo | Camptothecin (8 mg/kg) displays complete growth inhibition and regression in mice xenografts of various tumors, including colon, lung, breast, stomach, and ovary tumors. [3] In mice, combinations of Camptothecin (50 mg/kg) and TNF (5 and 7 μg/kg), but not Camptothecin alone, induces liver damage. [4] | |
|---|---|---|
| Animal Research | Animal Models | Nude mice (NIH-I high fertility strain) bearing xenografts of CASE, SW48, DOY, SPA, and CLO cells |
| Dosages | 0–8 mg/kg | |
| Administration | Administered via i.m. or i.v. injection | |
References |
|
Chemical Information
| Molecular Weight | 348.35 | Formula | C20H16N2O4 |
| CAS No. | 7689-03-4 | SDF | Download Camptothecin (CPT) SDF |
| Synonyms | Campathecin, (S)-(+)-Camptothecin,NSC-100880 | ||
| Smiles | CCC1(C2=C(COC1=O)C(=O)N3CC4=CC5=CC=CC=C5N=C4C3=C2)O | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
0.1M NaOH : 2.5 mg/mL DMSO : Insoluble ( Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field





































