research use only
BrdU (Bromodeoxyuridine) DNA/RNA Synthesis chemical
Cat.No.S7918
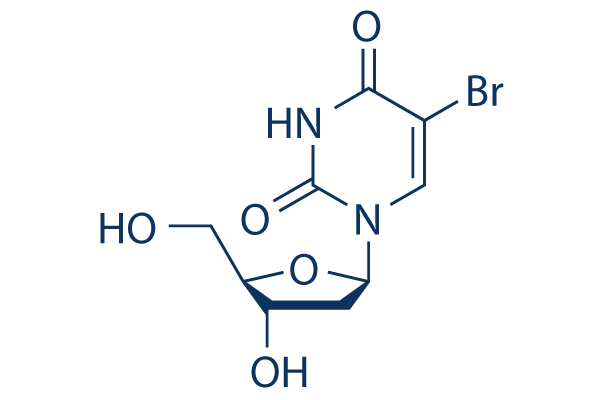
Chemical Structure
Molecular Weight: 307.1
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Triapine (3-AP) Carmofur YK-4-279 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| mouse neural precursor cells | Antiproliferative assay | Antiproliferative activity against mouse neural precursor cells by MTT assay, EC50=2.045μM | 17417631 | |||
| murine leukemia cells | Growth inhibition assay | In vitro growth inhibition of FdUrd resistant L5178Y-Resistant murine leukemia cells, IC50=12μM | 11101356 | |||
| murine leukemia cells | Growth inhibition assay | In vitro growth inhibition of L5178Y-Parental murine leukemia cells by incorporation of [14C]Leu., IC50=20μM | 11101356 | |||
| murine leukemia cells | Growth inhibition assay | In vitro growth inhibition of L5178Y-Parental murine leukemia cells, IC50=26μM | 11101356 | |||
| murine leukemia cells | Growth inhibition assay | In vitro growth inhibition of L5178Y-Parental murine leukemia cells by incorporation of [14C]-Thd., IC50=26μM | 11101356 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 61 mg/mL
(198.63 mM)
Ethanol : 3 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 307.1 | Formula | C9H11BrN2O5 |
Storage (From the date of receipt) | 3 years -20°C(in the dark) powder |
|---|---|---|---|---|---|
| CAS No. | 59-14-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | 5-Bromo-2'-deoxyuridine, BUdR | Smiles | C1C(C(OC1N2C=C(C(=O)NC2=O)Br)CO)O | ||
Mechanism of Action
| In vitro |
Bromodeoxyuridine (BrdU) induces a progressive, dose-responsive suppression of cancer cell line and cancer stem cell population expansion in RG2 rat glioma cells, and alters the cell cycle profile in H9 cells and BJ fibroblasts. This compound is stably integrated into the DNA, and thus can be used in assessment of cell proliferation and other cell procession. |
|---|---|
| In vivo |
In rat glioma RG2 tumor model, BrdU (300 mg/kg, i.p. or 0.8 mg/ml, p.o.) significantly slows tumor progression. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04365205 | Recruiting | Asthma |
University Hospital Bordeaux |
September 23 2020 | -- |
| NCT02654925 | Completed | Obesity |
University of Colorado Denver |
March 2016 | -- |
| NCT01407211 | Unknown status | Relapsing Remitting Multiple Sclerosis |
Tehran University of Medical Sciences |
April 2011 | Phase 4 |
| NCT01414972 | Unknown status | Atherosclerosis |
Tehran University of Medical Sciences |
May 2010 | Phase 4 |
| NCT01225289 | Completed | Relapsing Remitting Multiple Sclerosis |
Tehran University of Medical Sciences |
October 2009 | Phase 4 |
| NCT00032344 | Completed | Colorectal Cancer |
US Department of Veterans Affairs|VA Office of Research and Development |
October 1993 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































