research use only
Articaine HCl NF-κB inhibitor
Cat.No.S3150
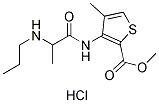
Chemical Structure
Molecular Weight: 320.84
Quality Control
Solubility
|
In vitro |
DMSO
: 64 mg/mL
(199.47 mM)
Water : 64 mg/mL Ethanol : 64 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 320.84 | Formula | C13H20N2O3S.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 23964-57-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Ultracaine | Smiles | CCCNC(C)C(=O)NC1=C(SC=C1C)C(=O)OC.Cl | ||
Mechanism of Action
| In vivo |
The time to maximum drug concentrations of articaine occurs about 10 to 15 minutes after submucosal injection of articaine 4% 80 mg. The elimination half-time of articaine is about 20 minutes. Articaine is better able to diffuse through soft tissue and bone than other local anaesthetics, the concentration of articaine in the alveolus of a tooth in the upper jaw after extraction is about 100 times higher than that in systemic circulation. Articaine: VAS (Visual Analogue Scale) scores (from 0 to 10 cm) by patients 4 to < 13 years of age are 0.5 for simple procedures and 1.1 for complex procedures, and average investigator scores are 0.4 and 0.6 for simple and complex procedures, respectively. No serious adverse events related to the articaine occurres, the only adverse event considered related to articaine is accidental lip injury in one patient. Articaine results in success rate of 64.5% in electronic pulp testing in healthy adult volunteers injected with 4% articaine. Articaine infiltration produces significantly more episodes of no response to maximum stimulation in first molars than lidocaine. Mandibular buccal infiltration is more effective with 4% articaine with epinephrine compared to 2% lidocaine with epinephrine. Articaine (4%) results in the success rate of 24% for the inferior alveolar nerve block in randomized, double-blind study. Articaine formulation results in successful pulpal anesthesia ranged from 75 to 92 percent and onset of pulpal anesthesia ranged from 4.2 to 4.7 minutes in healthy volunteer. For articaine, 4 percent (two of 56) of the subjects reported swelling and no subjects reported bruising. Ninety-eight percent (59 of 60) of the subjects had lip numbness with the articaine solution.
|
References |
|
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04759339 | Completed | Healthy |
American Genomics LLC |
February 24 2021 | Phase 1 |
| NCT04865848 | Terminated | Anesthesia |
University of Illinois at Chicago |
June 5 2019 | Not Applicable |
| NCT03174860 | Completed | Pulpitis - Irreversible|Anesthesia Local |
Cairo University |
October 2016 | Phase 2|Phase 3 |
| NCT01496846 | Completed | Irreversible Pulpitis |
University of Michigan|Dentsply International |
September 2011 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































