- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
2,2,2-Tribromoethanol
Cat.No.S4508
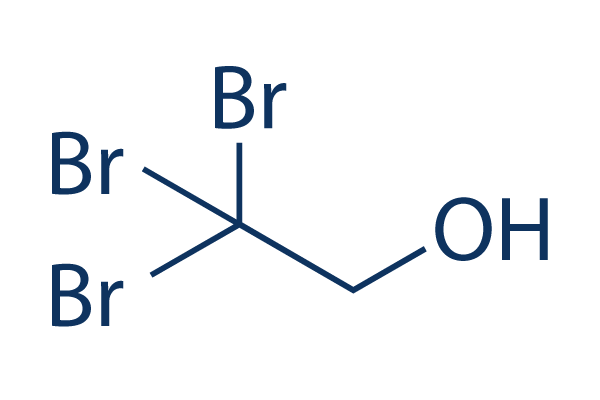
Chemical Structure
Molecular Weight: 282.76
Jump to
Quality Control
Solubility
|
In vitro |
DMSO
: 57 mg/mL
(201.58 mM)
Ethanol : 57 mg/mL Water : 29 mg/mL |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 282.76 | Formula | C2H3Br3O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 75-80-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | TBE, Tribromoethanol | Smiles | C(C(Br)(Br)Br)O | ||
Mechanism of Action
| In vivo |
Tribromoethanol has been proposed as an acceptable anaesthetic in dogs, cats, mice, rats, and gerbils. It possesses a concentration dependent irritant effect. When used at low concentration, it displays good anesthetic effect. A high dose of tribromoethanol is not a suitable anesthetic for major surgery in all mouse strains because of the risk of pathologic changes in the abdominal organs, such as retention of the digestive tract, peritonitis, and fibrinoid adhesion.
|
References |
|
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04573205 | Not yet recruiting | Tick-borne Encephalitis|Vaccine |
Region Örebro County |
January 2024 | Phase 4 |
| NCT05607394 | Recruiting | Tick-borne Encephalitis |
University Hospital Strasbourg France |
October 24 2022 | -- |
| NCT05138055 | Recruiting | Tick-Borne Encephalitis European |
Sykehuset Telemark|Sykehuset i Vestfold HF|Norwegian Institute of Public Health|Sorlandet Hospital HF|Oslo University Hospital |
October 1 2020 | -- |
| NCT03932448 | Unknown status | Tick-Bite; Fever|Tick Fever|Tick-Borne Diseases|Encephalitis Tick-Borne|Tick Bites |
Göteborg University|Sahlgrenska University Hospital Sweden|Vastra Gotaland Region |
May 15 2019 | -- |
| NCT03971058 | Unknown status | Japanese Encephalitis Vaccine |
Medical University of Vienna|Valneva Austria GmbH |
March 1 2019 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































