- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Ondansetron HCl 5-HT Receptor antagonist
Cat.No.S1390
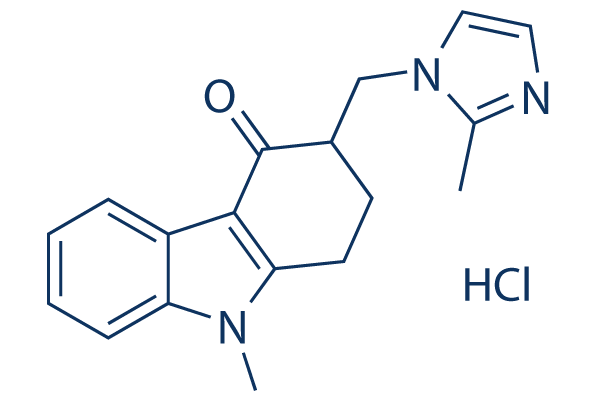
Chemical Structure
Molecular Weight: 329.82
Jump to
Quality Control
Batch:
Purity:
99.88%
99.88
Solubility
|
In vitro |
Water : 166 mg/mL
DMSO
: 66 mg/mL
(200.1 mM)
Ethanol : 13 mg/mL |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 329.82 | Formula | C18H19N3O.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 99614-01-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GR 38032F,SN 307 | Smiles | CC1=NC=CN1CC2CCC3=C(C2=O)C4=CC=CC=C4N3C.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
5-HT3
|
|---|---|
| In vivo |
Ondansetron decreases the intensity of withdrawal signs such as increased defecation, jumping and wet-dog shakes, elevated the nociceptive threshold values which are decreased by precipitated withdrawal, but produces no change in urination, rectal temperature or salivation. Ondansetron and granisetron significantly enhances gastric emptying of glass beads and improves cisplatin-induced slowing of gastric emptying in rats. Ondansetron exhibits a biphasic dose-response profile in mice, with antidepressant-like effects peaking at 0.1 mg/kg, in the forced swim and tail suspension tests. Ondansetron pretreatment augments the antidepressant effects of fluoxetine and venlafaxine but does not influence the effects of desipramine or 8-hydroxy-2-(di-n-propylamino) tetralin. Ondansetron (10 mg/kg) reverses hyperactivity in the open field, and decreases the percentage entry and time spent in open arms in the elevated plus maze. Ondansetron, a selective and potent 5HT3 receptor antagonist, is shown to be effective at blocking the amphetamine-induced disruption of LI at a dose of 0.01 mg/kg, but not at 0.1 mg/kg. Ondansetron is able to attenuate increases in dopamine activity, produces pharmacologically with amphetamine without affecting baseline dopamine activity. Ondansetron facilitates performance in young adult and aged animals, and inhibits an impairment in habituation induced by scopolamine, electrolesions or ibotenic acid lesions of the nucleus basalis magnocellularis. Ondansetron and arecoline antagonizes a scopolamine-induced impairment. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05759481 | Recruiting | Post-operative Nausea and Vomiting |
Milton S. Hershey Medical Center |
February 1 2024 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































