- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
Ibuprofen (NSC 256857) COX inhibitor
Ibuprofen (NSC 256857, Dolgesic) is an anti-inflammatory inhibitor targeting COX-1 and COX-2 with IC50 of 13 μM and 370 μM, respectively.
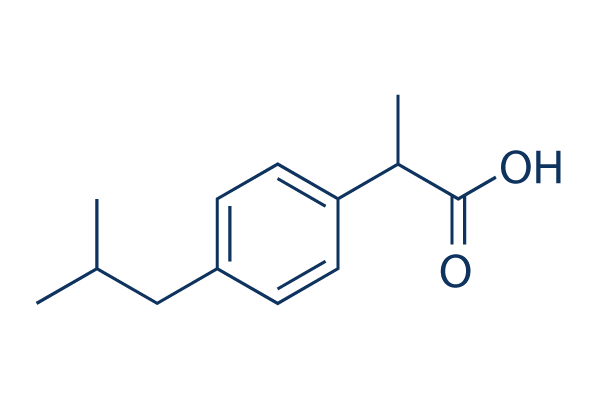
Chemical Structure
Molecular Weight: 206.28
Purity & Quality Control
Related Products
| Related Targets | COX-1 COX-2 | Click to Expand |
|---|---|---|
| Related Products | NS-398 (NS398) Paeoniflorin (NSC 178886) Lumiracoxib Xanthohumol Bufexamac Oroxin B Tolfenamic Acid Nimesulide Ginsenoside Rd Indometacin Sodium Pranoprofen (-)-Epicatechin gallate Salicin SC-560 Triflusal Phenacetin (+/-)-Sulfinpyrazone (+)-Catechin hydrate Niflumic acid Etoricoxib | Click to Expand |
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Neuronal Signaling Compound Library CNS-Penetrant Compound Library Anti-alzheimer Disease Compound Library | Click to Expand |
Signaling Pathway
Mechanism of Action
| Features | Considered a core medicine in the WHO's "WHO Model List of Essential Medicines" (a list of the minimum medical requirements for a basic healthcare system). | ||||
|---|---|---|---|---|---|
| Targets |
|
In vitro |
||||
| In vitro | Ibuprofen (NSC 256857) works by inhibiting the enzyme cyclooxygenase COX-1 and COX-2, which convert arachidonic acid to prostaglandin H2 (PGH2). Its action is similar to aspirin, indomethacin and all other NSAIDs in intact cells, broken cells, and purified enzyme preparations. [1] This compound inhibits the constitutive activation of NF-κB and IKKα in the androgen-independent prostate tumor cells PC-3 and DU-145. It sensitizes prostate cells to ionizing radiation and blocks stimulated activation of NF-κB following exposure to TNFα or ionizing radiation in the androgen-sensitive prostate tumor cell line LNCaP. Both of these cannot be attributed directly to inhibition of IκB-α kinase but to inhibition of an upstream regulator of IKKα. [2] It exerts an anticancer effect by reducing survival of cancer cells. Ibuprofen is more efficacious than aspirin and acetaminophen, and comparable with (R)-flurbiprofen and indomethacin in induction of p75NTR protein (a tumor and metastasis suppressor) expression in cell lines from bladder and other organs. [3] | |||
|---|---|---|---|---|
| Kinase Assay | Radiochemical enzyme assays for COX-1 and COX-2 | |||
| 10 μL of purified COX-1 (0.7-0.8 μg) or COX-2 (3.0 units, 0.3μg) is activated with 50 μL of cofactor solution [l-epinephrine (1.3 mg/mL), reduced glutathione (0.3 mg/mL), and hematin (1.3 mg/mL) in oxygen-free Tris-HCl buffer (pH 8.0)]. After [14C]arachidonic acid is added in 0.2 mL eight-strip test tubes and preincubated 10 minutes on ice, the enzyme solution (60 μL) is added to Ibuprofen (NSC 256857) solutions or DMSO (20 μL). Samples are incubated for 15 minutes at 37 °C, after which the reaction is terminated by addition of 10 μL of 2 M HCl and 5 μL of carrier solution (PGE2 and PGF2α, 0.2 μg/mL of each in EtOH). The unmetabolized arachidonic acid is separated from the prostaglandin products by column chromatography and eluted with n-hexane-dioxane-glacial acetic acid (70:30:1). The prostaglandin products are then eluted with EtOAc-MeOH (85:15), and the samples are counted in a Packard scintillation spectrometer. IC50 values are obtained by linear regression analysis. | ||||
| Cell Research | Cell lines | Bladder epithelial cell line T24, RT-4 transitional cell papilloma bladder cell line, 5637 primary carcinoma bladder cell line, HCT-116, MDAMB231, MCF7, HEK293, A549, SKOV3 and DU145 | ||
| Concentrations | Dissolved in DMSO at a concentration of 200 mM for making the stock solution, final concentration ~2 mM | |||
| Incubation Time | 48 hours | |||
| Method | Each cell line is incubated with Ibuprofen (NSC 256857) of various concentrations for 48 hours. Cell survival is estimated by the MTT assay, and cell death is determined by Hoechst staining used to distinguish between intact cell nuclei and fragmented nuclei undergoing cell death. Cells are lysed and analyzed by western blotting for detection of the p75NTR protein. | |||
In Vivo |
||
| In vivo | Ibuprofen (NSC 256857) reacts with the heme group of cyclooxygenase to prevent arachidonic acid conversion. Prior exposure to this compound in vivo protects cyclooxygenase completely from the irreversible effects of aspirin in platelets. [4] Its treatment is effective in attenuating joint inflammation and early articular cartilage degeneration in the adult female Sprague-Dawley rat model induced by high-repetition and high-force (HRHF) task. It does this by blocking the increases in serum C1 and 2C (a biomarker of collagen I and II degradation) as well as the ratio of collagen degradation to synthesis (C1, 2C/CPII, the latter a biomarker of collage type II synthesis) induced by HRHF. [5] | |
|---|---|---|
| Animal Research | Animal Models | Female Sprague-Dawley rats with joint inflammation induced by high-repetition and high-force (HRHF) tasks |
| Dosages | 45 mg/kg | |
| Administration | Taken orally every day | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06146491 | Recruiting | Pain Postoperative |
B.P. Koirala Institute of Health Sciences |
August 10 2023 | Phase 4 |
| NCT05928520 | Not yet recruiting | Pain Management |
Al-Azhar University |
August 1 2023 | Phase 3 |
| NCT06247462 | Completed | Acute Kidney Injury |
University of New Mexico |
June 1 2023 | Phase 1 |
| NCT05575700 | Recruiting | Pain Acute|Hip Arthropathy|Knee Arthropathy|Safety Issues|Analgesia|Analgesic Adverse Reaction|Postoperative Pain|Postoperative Complications |
Naestved Hospital |
April 17 2023 | Phase 4 |
| NCT05496868 | Recruiting | Acute Respiratory Distress Syndrome Adult |
Dompé Farmaceutici S.p.A |
February 7 2023 | Phase 2 |
References |
|
Chemical Information
| Molecular Weight | 206.28 | Formula | C13H18O2 |
| CAS No. | 15687-27-1 | SDF | Download SDF |
| Synonyms | Dolgesic,NSC 256857 | ||
| Smiles | CC(C)CC1=CC=C(C=C1)C(C)C(=O)O | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 41 mg/mL ( (198.75 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 41 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field