- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Volasertib
PLK inhibitor
research use only
Volasertib is a highly potent Plk1 inhibitor with IC50 of 0.87 nM in a cell-free assay. It shows 6- and 65-fold greater selectivity against Plk2 and Plk3. Volasertib induces cell cycle arrest and apoptosis in various cancer cells. Phase 3.
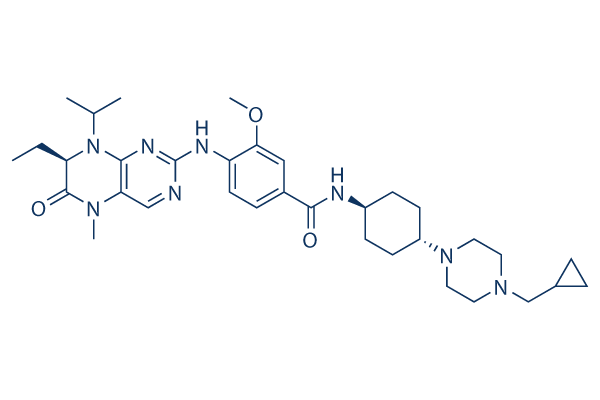
Chemical Structure
Molecular Weight: 618.81
Purity & Quality Control
Batch:
Purity:
99.78%
99.78
Related Products
| Related Targets | PLK1 PLK2 PLK3 PLK4 | Click to Expand |
|---|---|---|
| Related Products | BI 2536 Rigosertib (ON-01910) GSK461364 Onvansertib (NMS-P937) SBE 13 HCl Ro3280 CFI-400945 HMN-214 MLN0905 Poloxin | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library PI3K/Akt Inhibitor Library MAPK Inhibitor Library DNA Damage/DNA Repair compound Library Cell Cycle compound library | Click to Expand |
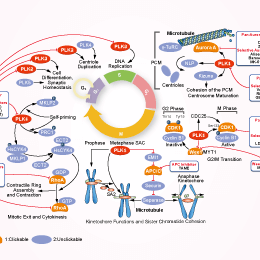
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| KASUMI-1 | Growth Inhibition Assay | 72 h | IC50=170±51 nM | 25576074 | ||
| KG-1 | Growth Inhibition Assay | 72 h | IC50=150±67 nM | 25576074 | ||
| MOLM-13 | Growth Inhibition Assay | 72 h | IC50=57±44 nM | 25576074 | ||
| MV-4-11 | Growth Inhibition Assay | 72 h | IC50=16±6 nM | 25576074 | ||
| NOMO-1 | Growth Inhibition Assay | 72 h | IC50=145±7 nM | 25576074 | ||
| OCI-AML3 | Growth Inhibition Assay | 72 h | IC50=90±51 nM | 25576074 | ||
| SKM-1 | Growth Inhibition Assay | 72 h | IC50=95±52 nM | 25576074 | ||
| THP-1 | Growth Inhibition Assay | 72 h | IC50=56±39 nM | 25576074 | ||
| MCF7/LTED | Growth Inhibition Assay | 2.5-40 nM | 5 d | inhibits cell growth in a dose-dependent manner | 25480943 | |
| HCC1428/LTED | Growth Inhibition Assay | 2.5-40 nM | 5 d | inhibits cell growth in a dose-dependent manner | 25480943 | |
| A431 | Growth Inhibition Assay | 0-30 nM | 1-4 d | inhibits cell growth in both dose- and time-dependent manner | 23891096 | |
| FaDu | Growth Inhibition Assay | 0-1000 nM | 1-4 d | inhibits cell growth in both dose- and time-dependent manner | 23891096 | |
| SF188 | Growth Inhibition Assay | 50-150 nM | 72 h | DMSO | inhibits cell proliferation | 23887645 |
| T98G | Growth Inhibition Assay | 50-150 nM | 72 h | DMSO | inhibits cell proliferation | 23887645 |
| DU145 | Growth Inhibition Assay | 10/50/250 nM | 24 h | IC50<10 nM | 23884428 | |
| LNCaP | Growth Inhibition Assay | 10/50/250 nM | 24 h | IC50<10 nM | 23884428 | |
| PC3 | Growth Inhibition Assay | 10/50/250 nM | 24 h | IC50∼600 nM | 23884428 | |
| RT4 | Growth Inhibition Assay | 48 h | IC50=111.27 nM | 23792639 | ||
| 5637 | Growth Inhibition Assay | 48 h | IC50=1165.14 nM | 23792639 | ||
| T24 | Growth Inhibition Assay | 48 h | IC50=204.91 nM | 23792639 | ||
| KMCH-1 | Apoptosis Assay | 200 nM | 24 h | induces apoptosis | 23703673 | |
| Mz-ChA-1 | Apoptosis Assay | 200 nM | 24 h | induces apoptosis | 23703673 | |
| HUCCT-1 | Apoptosis Assay | 200 nM | 24 h | induces apoptosis | 23703673 | |
| HCT 116 | Growth Inhibition Assay | EC50 = 23 nM | 19383823 | |||
| NCI-H460 | Growth Inhibition Assay | EC50 = 21 nM | 19383823 | |||
| BRO | Growth Inhibition Assay | EC50 = 11 nM | 19383823 | |||
| GRANTA-519 | Growth Inhibition Assay | EC50 = 15 nM | 19383823 | |||
| HL-60 | Growth Inhibition Assay | EC50 = 32 nM | 19383823 | |||
| THP-1 | Growth Inhibition Assay | EC50 = 36 nM | 19383823 | |||
| Raji | Growth Inhibition Assay | EC50 = 37 nM | 19383823 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| SKBR3 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human SKBR3 cells after 24 hrs by MTT assay | 29288948 | ||
| MDA-MB-231 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MDA-MB-231 cells after 24 hrs by MTT assay | 29288948 | ||
| MDA-MB-468 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MDA-MB-468 cells after 24 hrs by MTT assay | 29288948 | ||
| BT474 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human BT474 cells after 24 hrs by MTT assay | 29288948 | ||
| ZR-75-1 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human ZR-75-1 cells after 24 hrs by MTT assay | 29288948 | ||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Features | A high volume of distribution, indicating good tissue penetration, and a long terminal half-life. | ||
|---|---|---|---|
| Targets |
|
In vitro |
||||
| In vitro | Like BI2536, BI6727 is an ATP-competitive kinase inhibitor from the dihydropteridinone class of compounds. In addition to Plk1, BI6727 also potently inhibits two closely related kinases Plk2 and Plk3 with IC50 of 5 nM and 56 nM, respectively. BI6727 at concentrations up to 10 μM displays no inhibitory activity against a panel of >50 other kinases. BI6727 inhibits the proliferation of multiple cell lines derived from various cancer tissues, including HCT116, NCI-H460, BRO, GRANTA-519, HL-60, THP-1, and Raji cells with EC50 of 23 nM, 21 nM, 11 nM, 15 nM, 32 nM, 36 nM, and 37 nM, respectively. BI6727 treatment (100 nM) in NCI-H460 cells induces an accumulation of mitotic cells with monopolar spindles and positive staining for histone H3 phosphoserine 10, confirming that cells are arrested early in the M phase, followed by induction of apoptosis. [1] Low nanomolar concentrations of BI6727 display potent inhibitory activity against neuroblastoma (NB) tumor-initiating cells (NB TIC) with EC50 of 21 nM, whereas only micromolar concentrations of BI6727 are cytotoxic for normal pediatric neural stem cells. [2] BI6727 induces growth arrest of Daoy and ONS-76 medulloblastoma cells similar to BI 2536. [3] |
|||
|---|---|---|---|---|
| Kinase Assay | In vitro kinase assays | |||
| Recombinant human Plk1 (residues 1-603) is expressed as NH2-terminal, GST-tagged fusion protein using a baculoviral expression system and purified by affinity chromatography using glutathione-agarose. Enzyme activity assays for Plk1 are done in the presence of serially diluted BI6727 using 20 ng of recombinant kinase and 10 μg casein from bovine milk as substrate. Kinase reactions are done in a final volume of 60 μL for 45 minutes at 30 °C [15 mM MgCl2, 25 mM MOPS (pH 7.0), 1 mM DTT, 1% DMSO, 7.5 μM ATP, 0.3 μCi γ-32P-ATP]. Reactions are terminated by the addition of 125 μL of ice-cold 5% TCA. After transferring the precipitates to MultiScreen mixed ester cellulose filter plates, plates are washed with 1% TCA and quantified radiometrically. Dose-response curves are used for calculating IC50 value. | ||||
| Cell Research | Cell lines | HCT116, NCI-H460, BRO, GRANTA-519, HL-60, THP-1, and Raji | ||
| Concentrations | Dissolved in DMSO, final concentrations ~1 μM | |||
| Incubation Time | 24, 48, and 72 hours | |||
| Method | Cell proliferation assays are done by incubating cells in the presence of various concentrations of BI6727 for 24, 48, and 72 hours and cell growth is assessed by measuring Alamar blue dye conversion in a fluorescence spectrophotometer. Effective concentrations at which cellular growth is inhibited by 50% (EC50) are extrapolated from the dose-response curve fit. To determine the DNA content, cell suspensions are fixed in 80% ethanol, treated for 5 minutes with 0.25% Triton X-100 in PBS, and incubated with 0.1% RNase and 10 μg/mL propidium iodide in PBS for 20 minutes at room temperature. Cell cycle profiles are determined by flow cytometric analysis. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p-PLK1 / PLK1 p-AKT / AKT / p-MAPK / MAPK PARP / c-myc p-c-Met / c-Met / p-FAK / FAK / p-Src / Src Fibronectin / β-integrin / p-vimentin / Vimentin / p-HH3 |
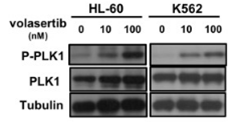
|
29108241 | |
| Immunofluorescence | PLK1 / Wee1 |
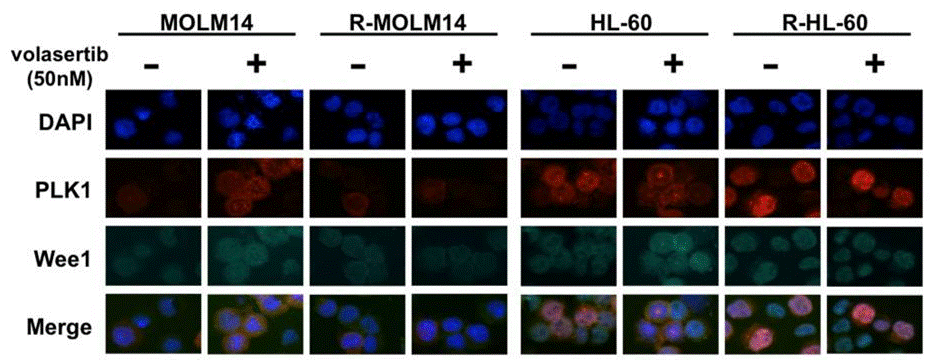
|
29108241 | |
| Growth inhibition assay | Cell viability |
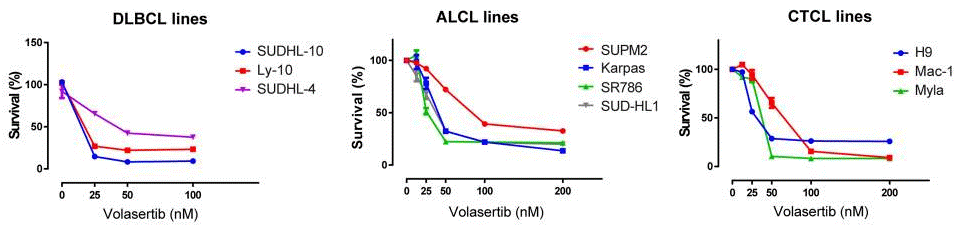
|
29383095 | |
In Vivo |
||
| In vivo | Administration of BI6727 significantly inhibits the growth of multiple human carcinoma xenografts including HCT116, NCI-H460, and taxane-resistant CXB1 colon carcinoma, accompanied by an increase in the mitotic index as well as an increase in apoptosis. [1] In in vivo studies, BI6727 shows better toxicity and pharmacokinetic profile compared to BI2536. [3] |
|
|---|---|---|
| Animal Research | Animal Models | Female BomTac:NMRI-Foxn1nu mice grafted s.c. with HCT116, NCI-H460, or CXB1 cells |
| Dosages | ~25 mg/kg/day | |
| Administration | Injected i.v., or given intragastrally via gavage needle | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02722135 | Withdrawn | Leukemia Myeloid Acute |
Boehringer Ingelheim |
November 2016 | Phase 1 |
| NCT02721875 | Terminated | Myelodysplastic Syndromes |
Boehringer Ingelheim |
April 28 2016 | Phase 1 |
| NCT02201329 | Completed | Myelodysplastic Syndromes|Leukemia Myelomonocytic Chronic |
Boehringer Ingelheim |
August 2014 | Phase 1 |
| NCT01971476 | Completed | Leukemia|Neoplasms |
Boehringer Ingelheim |
October 22 2013 | Phase 1 |
| NCT01772563 | Completed | Neoplasms |
Boehringer Ingelheim |
February 4 2013 | Phase 1 |
| NCT01662505 | Completed | Leukemia Myeloid Acute |
Boehringer Ingelheim |
August 2012 | Phase 1 |
References |
|
Chemical Information
| Molecular Weight | 618.81 | Formula | C34H50N8O3 |
| CAS No. | 755038-65-4 | SDF | Download SDF |
| Synonyms | BI 6727 | ||
| Smiles | CCC1C(=O)N(C2=CN=C(N=C2N1C(C)C)NC3=C(C=C(C=C3)C(=O)NC4CCC(CC4)N5CCN(CC5)CC6CC6)OC)C | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 35 mg/mL ( (56.56 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Frequently Asked Questions
Question 1:
I wonder how to reconstitute the inhibitor for in vivo studies?
Answer:
Volasertib can be dissolved in 4% DMSO+Corn oil at 2mg/ml for i.p. injection in mice. For oral administration, it can be formulated in hydrochloric acid (0.1 N), and diluted with 0.9% NaCl, or suspended in 0.5% Natrosol 250 hydroxyethyl-cellulose as indicated in the publications. We also suggest the vehicle 30% PEG400/0.5% Tween80/5% propylene glycol for a suspension which we tested in house.