Polo-like kinases (PLKs) are a family of evolutionarily conserved Serine/Threonine kinases, which are characterized by the presence of an N-terminal protein kinase domain and a C-terminal Polo-box domain (PBD), playing an important role in the regulation of cell cycle progression through G2 and M (mitosis). [show the full text]
PLK inhibitors
| Cat.No. | Product Name | Information | Product Use Citations | Product Validations |
|---|---|---|---|---|
| S2235 | Volasertib (BI6727) | Volasertib is a highly potent Plk1 inhibitor with IC50 of 0.87 nM in a cell-free assay. It shows 6- and 65-fold greater selectivity against Plk2 and Plk3. Volasertib induces cell cycle arrest and apoptosis in various cancer cells. Phase 3. |
|
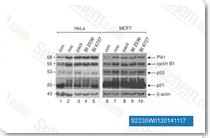
|
| S1109 | BI 2536 | BI-2536 is a potent Plk1 inhibitor with IC50 of 0.83 nM in a cell-free assay. BI-2536 inhibits Bromodomain 4 (BRD4) with Kd of 37 nM and potently suppresses c-Myc expression. BI-2536 induces apoptosis and attenuates autophagy. Phase 2. |
|
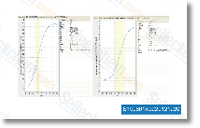
|
| S1362 | Rigosertib (ON-01910) | Rigosertib (ON-01910) is a non-ATP-competitive inhibitor of PLK1 with IC50 of 9 nM in a cell-free assay, showing 30-fold greater selectivity against Plk2 and no activity to Plk3. It inhibits the PI3K/Akt pathway, activates oxidative stress signals, and induces apoptosis in various cancer cells. This compound is in Phase 3. |
|
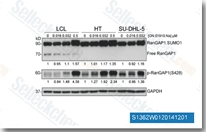
|
| S2193 | GSK461364 | GSK461364 (GSK461364A) inhibits purified Plk1 with Ki of 2.2 nM in a cell-free assay. It is more than 1000-fold selective against Plk2/3. |
|
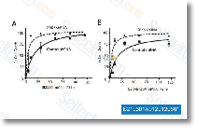
|
| S7255 | Onvansertib (NMS-1286937, NMS-P937) | Onvansertib (NMS-1286937, NMS-P937) is an orally available, selective Polo-like Kinase 1 (PLK1) inhibitor with IC50 of 2 nM, 5000-fold selectivity over PLK2/PLK3. This compound potently causes a mitotic cell-cycle arrest followed by apoptosis in cancer cell lines and inhibits tumor growth. Phase 1. |
|
|
| S7720 | SBE 13 HCl | SBE 13 HCl is a potent and selective PLK1 inhibitor with IC50 of 200 pM, >4000-fold selectivity over Aurora A kinase, Plk2 and Plk3. |
|
|
| S1485 | HMN-214 | HMN-214 is a prodrug of HMN-176, which alters the cellular spatial orientation of Plk1. |
|
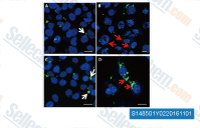
|
| S7248 | Ro3280 | RO3280 (Ro5203280) is a potent, highly selective inhibitor of Polo-like kinase 1 (PLK1) with IC50 of 3 nM. |
|
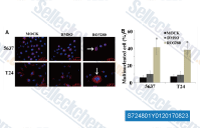
|
| S7552 | CFI-400945 | CFI-400945 is an orally active, potent and selective polo-like kinase 4(PLK4) inhibitor with Ki value of 0.26 nM. |
|
|
| S2898 | MLN0905 | MLN0905 is a potent inhibitor of PLK1 with IC50 of 2 nM. |
|
Signaling Pathway Map
PLKs - the Serine/Threonine protein kinases - are structurally comprised of a highly conserved catalytic domain in the N-terminus and a PBD in the C-terminus. The PDB domain is unique for the polo-like kinases family, and essential for their functions such as the subcellular localization and the substrate recognition. Mammalian polo-like kinases (PLK) consists of five members including PLK1 (STPK13), PLK2 (SNK), PLK3 (CNK, FNK or PRK), PLK4 (SAK or STK18), and PLK5. PLKs are critical regulators of cell cycle progression, mitosis, cytokinesis, and the DNA damage response. [1][2]
PLK1 is the most extensively studied member of this family. Through its PBD, PlK1 associates with a large number of proteins, some of which mediate its recruitment to defined cellular structures, such as the centrosomes, kinetochores and the spindle midzone. As reflected by multiple localizations, PLK1 is pivotal in the regulation of diverse cellular and biochemical events at multiple stages of the M phase, including entry into mitosis, centrosome maturation, assembly of the bipolar spindle, sister chromatid splitting, activation of the anaphase-promoting complex/cyclosome (APC/C) and exit from mitosis with the initiation of cytokinesis. As PLK1 is essential for cell division, inhibition of PLK1 leads to a failure to complete mitosis, eventually resulting in cell death. PLK2 is classified as an early growth response gene by virtue of its increased expression on stimulation by growth factors. PLK2 is up-regulated by p53 upon irradiation, and the depletion of PLK2 seems to phenocopy the loss of p53 by elevating the sensitivity of cells to spindle poisons. PLK2 is also implicated in centriole duplication, and functions as a regulator of synaptic plasticity in the nervous system. PLK3 is a multifunctional stress response protein. In response to different types of cellular stress, PLK3 activates Chk2 and p53, and plays an important role in genotoxic, hypoxic and oxidative stress responses. Endogenous ectopically expressed PLK3 localizes to the centrosomes during interphase, to the spindle poles during mitosis, and to the midbody during cytokinesis. The overexpression of wild-type or mutant forms of PLK3 induces G2/M arrest and eventually apoptosis. PlK4 is crucial for the duplication of the centrioles, and essential for early embryogenesis. The overexpression of PLK4 induces multinucleated cells that are associated with a progressive loss of cell viability. Although PLK5 has a truncated kinase domain that lacks kinase activity of catalytic activity, PLK5 retains important functions in neuron biology. Overexpression of PLK5 induces G1 cell cycle arrest followed by apoptosis. PLK5 can be induced by various stress-promoting agents, which is independent of p53 in contrast to PLK2 and PLK3. [1][2][3]
Polo-like kinases are dysregulated in a variety of human cancers. Consistent with the critical functions of PLK1 during M-phase progression, PLK1 is highly expressed in tumors including those derived from lung, breast, colon, pancreas, prostate and ovary. Over-expression of PLK1 correlates with cellular proliferation and poor prognosis, and PLK1 depletion is associated with a decrease in cell viability and induction of apoptosis in various cancerous cells, indicating that PLK1 is a promising target in oncology. Small-molecule inhibitors of PLK1 have become attractive candidates for anticancer drug development targeting the kinase domain and the PBD of PLK1, and a variety of PLK1 inhibitors (GW843682X, BI 2536, et al.) have been clinically assessed and hold great promise for the improved treatment of cancer patients. [1][2]





































