- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Mycophenolate mofetil Dehydrogenase inhibitor
Cat.No.S1501
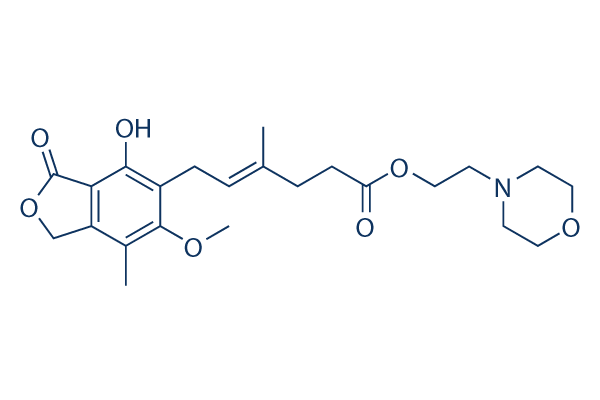
Chemical Structure
Molecular Weight: 433.49
Jump to
Quality Control
Batch:
Purity:
99.98%
99.98
Solubility
|
In vitro |
DMSO
: 87 mg/mL
(200.69 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 433.49 | Formula | C23H31NO7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 128794-94-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Mycophenolic acid morpholinoethyl ester, CellCept, RS-61443, TM-MMF | Smiles | CC1=C2COC(=O)C2=C(C(=C1OC)CC=C(C)CCC(=O)OCCN3CCOCC3)O | ||
Mechanism of Action
| Targets/IC50/Ki |
Inosine monophosphate dehydrogenase II
(Cell-free assay) 27 nM
Inosine monophosphate dehydrogenase I
(Cell-free assay) 39 nM
|
|---|---|
| In vitro |
Mycophenolate mofetil is an ester prodrug of the active immunosuppressant mycophenolic acid (MPA). The latter shows a noncompetitive, selective and reversible inhibition activity against inosine monophosphate dehydrogenase type I/II with IC50 of 39 nM and 27 nM, respectively. Moreover, MPA also produces the concentration-dependent inhibition of proliferation of ConA-stimulated T cells, LPS-stimulated B cells and alloantigen-specific T cells with IC50 of 100 nM, 120 nM, and 51 nM, respectively. This compound with high concentration of 10 μg/mL induces a strong apoptosis in microglial cell cultures and increases the number of activated caspase-3 immunoreactive apoptotic cells. In addition, it (1 μg/mL) strongly inhibits proliferation of both microglial cells and astrocytes. A recent study shows that this chemical significantly attenuates the extent of neuronal cell death of organotypic hippocampal slice cultures after neuronal injury in a time-dependent manner. |
| Kinase Assay |
IMP dehydrogenase Types I and II enzymatic activity
|
|
IMP dehydrogenase Types I and II are purified from E. coli expressing human enzymes. The assay is performed using a flat bottom, UV-transparent 96-well plate. The final 200 μL reaction mixture contained 0.1 M Tris, 0.1 M KCl, 3 mM EDTA pH 8.0, 2 mM DTT, and 40 nM of either IMP dehydrogenase Type I or Type II. The reaction is initiated by adding 400 μM NAD and 400 μM IMP, followed by incubation at 37 °C for 2.5 hours. The reaction rate of the conversion of NAD to NADH is then measured based on the increase in absorbance at 340 nm. The assays are also performed in the presence of 50% human serum to estimate serum protein binding by different IMP dehydrogenase inhibitors.
|
|
| In vivo |
In an ACI-to-Lewis rat heterotopic cardiac transplant model, treatment of Mycophenolate mofetil at doses of 20 mg/kg and 40 mg/kg leads to a prolongation of graft survival, with median survival time (MST) of 14.5 days and 18.5 days, respectively. In bleomycin (BLM)-induced scleroderma mouse model, this compound reduces inflammatory-cell infiltration, tissue hydroxyproline content and dermal thickness. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05702931 | Not yet recruiting | Hyperglycemia|Renal Transplant Complication Primary Non-Function|Diabetes |
Rigshospitalet Denmark|Aarhus University Hospital|Odense University Hospital |
April 1 2024 | Phase 4 |
| NCT05859191 | Recruiting | Systemic Lupus Erythematosus|Physiopathology |
University Hospital Tours|Research Center for Respiratory Diseases Inserm U1100 |
July 21 2023 | -- |
| NCT05120570 | Recruiting | Acute Leukemia|Myelodysplastic Syndromes|Myeloproliferative Neoplasm|Lymphoma |
Masonic Cancer Center University of Minnesota |
March 17 2022 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































