- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Indomethacin COX inhibitor
Cat.No.S1723
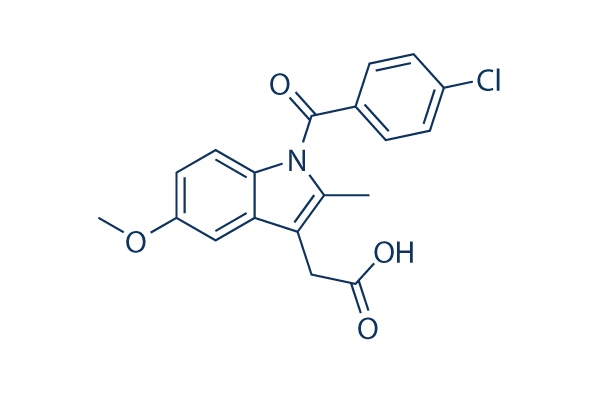
Chemical Structure
Molecular Weight: 357.79
Jump to
Quality Control
Batch:
Purity:
100.00%
100.00
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HeLa cells | Function assay | 20 mins | Agonist activity at androgen receptor (unknown origin) expressed in HeLa cells co-expressing PSA-(ARE)4-Luc13 assessed as induction of DHT-induced luciferase activity after 20 mins by luciferase reporter gene assay, EC50=0.14 nM | |||
| J774 cells | Function assay | In vitro inhibitory activity against Prostaglandin G/H synthase 1 in murine J774 cells, IC50=2 nM | ||||
| U-937 cells | Function assay | In vitro inhibitory activity against human Prostaglandin G/H synthase 1 (COX-1) in U-937 cells, IC50=2 nM | ||||
| rat synovial cells | Function assay | In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells, IC50=2.9 nM | ||||
| 143982 cells | Function assay | In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (COX-2) in 143982 cells, IC50=9 nM | ||||
| CHO cells | Function assay | In vitro inhibitory potency against human COX-1 in stably transfected chinese hamster ovary (CHO) cells, IC50=18 nM | ||||
| RBL-1 cell line | Function assay | Inhibition of Prostaglandin G/H synthase in intact RBL-1 cell line, IC50=0.5 μM | ||||
| SF-9 insect cells | Function assay | 20 Mins | Inhibition of human COX2 expressed in SF-9 insect cells assessed as conversion of [14C]-arachidonic acid to [14C]-prostaglandins preincubated for 20 mins by thin-layer chromatography analysis, IC50=0.75 μM | |||
| mouse RAW264.7 cells | Function assay | 24 h | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced NO production after 24 hrs by Griess assay, IC50=1.25 μM | |||
| mouse BV2 cells | Function assay | 24 h | Antiinflammatory activity in mouse BV2 cells assessed as inhibition of LPS-induced NO production after 24 hrs by Griess assay, IC50=7.1 μM | |||
| human 2008 cells | Function assay | Inhibition of human MRP1 in human 2008 cells, IC50=12 μM | ||||
| A549 cells | Function assay | 10 μM | Inhibition of mPGES1 in human A549 cells assessed as inhibition of IL-1-beta-induced PGE2 formation at 10 uM by cell-intact assay | |||
| HepG2 cells | Function assay | 100 μM | Inhibition of MRP1-mediated doxorubicin efflux in doxorubicin resistant human HepG2 cells at 100 uM by flow cytometry | |||
| THP1 cells | Function assay | 100 μM | 30 mins | Irreversible inhibition of COX-1 in human THP1 cells assessed as inhibition of arachidonic acid-induced TXB2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | ||
| MDA-MB-231 cells | Function assay | 100 μM | 30 mins | Irreversible inhibition of COX-1 in human MDA-MB-231 cells assessed as inhibition of arachidonic acid-induced PGE2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | ||
| LNCAP cells | Function assay | 30 μM | 48 h | Inhibition of AKR1C3 (unknown origin)-mediated testosterone-17beta-glucuronide formation expressed in human LNCAP cells at 30 uM after 48 hrs in presence of 4-androstene-3,17-dione | ||
| mouse BV2 cells | Cytotoxicity assay | 24 h | Cytotoxicity against mouse BV2 cells assessed as maximum non-toxic concentration after 24 hrs by MTT assay | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 72 mg/mL
(201.23 mM)
Ethanol : 24 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 357.79 | Formula | C19H16ClNO4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 53-86-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Indometacin,NSC-77541 | Smiles | CC1=C(C2=C(N1C(=O)C3=CC=C(C=C3)Cl)C=CC(=C2)OC)CC(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
COX1
0.28 μM
COX-2
14 μM
|
|---|---|
| In vitro |
Indomethacin inhibits transcription of a beta-catenin/TCF-responsive reporter gene in a dose dependent manner. This compound also downregulates the beta-catenin/TCF transcriptional target cyclin D1. It attenuates the transcription of beta-catenin/TCF-responsive genes, by modulating TCF activity without disrupting beta-catenin/TCF complex formation.
|
| In vivo |
Indomethacin increases BrdU+ cells of all lineages and reduces microglial/monocyte activation in rats after focal cerebral ischemia. This compound (7.5 mg/kg, one injection) produces acute injury and inflammation in the distal jejunum and proximal ileum that are maximal at three days and completely resolves within one week. It (two daily subcutaneous injections) produces a more extensive and chronic inflammation that lasts in an active form in more than 75% of the rats for at least two weeks. This chemical (4ppm) reduces tumor yield by 78% in SKH:HR-1-hrBr hairless mice. It (20 mg/kg, orally) increases the total area of gastric erosions and concentration of lipid peroxides in the gastric mucosa of rats. This compound increases the alpha-tocopherol:total cholesterol ratio in serum. It inhibits the increases in gastric mucosal erosions and lipid peroxides in the gastric mucosa, and the reduction of serum alpha-tocopherol.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06031363 | Completed | Pancreatitis|Cholangiopancreatography Endoscopic Retrograde|Indomethacin |
The First Affiliated Hospital of Soochow University |
November 1 2022 | Not Applicable |
| NCT04821323 | Completed | Healthy |
Janssen Research & Development LLC |
March 10 2021 | Early Phase 1 |
| NCT04025177 | Withdrawn | Patent Ductus Arteriosus After Premature Birth |
University of Manitoba|St. Boniface Hospital|Health Sciences Centre Winnipeg Manitoba|University at Buffalo |
January 2020 | Phase 2 |
| NCT03967483 | Unknown status | Headache Migraine |
Anne Ducros|French Society for the Study of Migraine Headache |
June 1 2019 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































