research use only
3-Methyladenine (3-MA) Autophagy/PI3K inhibitor
Cat.No.S2767
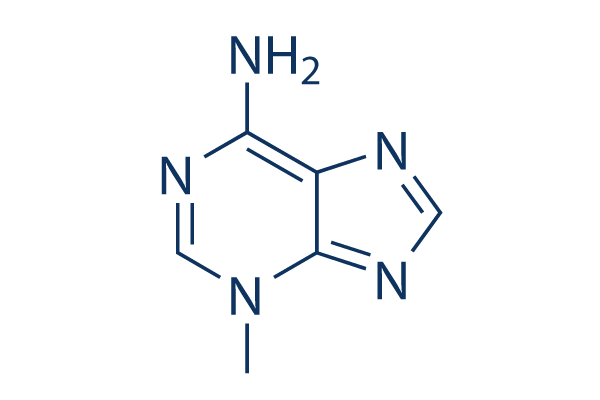
Chemical Structure
Molecular Weight: 149.15
Quality Control
Products Often Used Together with 3-Methyladenine (3-MA)
| Related Targets | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other PI3K Inhibitors | GDC-0077 (Inavolisib) SAR405 Quercetin (Sophoretin) LY294002 XL147 analogue Tersolisib (STX-478) Buparlisib (BKM120) 740 Y-P (PDGFR 740Y-P) GO-203 TFA Eganelisib (IPI-549) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| K562 | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| Jurkat | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| HeLa | Cytotoxicity Assay | 2mM | 24h | inhibites the cytotoxicity of silibinin to HeLa cells. | 21875385 | |
| PC12/TetOn | Function Assay | 0.1/1mM | 18h | leads to α-syn(WT) accumulation, toxicity, and oligomer formation | 21906659 | |
| RMPI8226 | Function Assay | 5mM | 1h | suppresses the level of autophagy under nutrient depletion | 21915620 | |
| MCF-7 | Function Assay | 10mM | 48h | blocks autophagy induced by bortezomib | 21931937 | |
| HBx | Apoptosis Assay | 10mM | 48h | DMSO | increases cell death | 22020078 |
| Marc-145 | Function Assay | 5mM | 12/24/36h | reduces the PRRSV titers and the protein expression | 22119900 | |
| U937 | Function Assay | 2mM | 12h | decreases the autophagy ratio | 22155150 | |
| BGC-823 | Function Assay | 5mM | 2h | inhibits the rate of autophagic cells | 22322152 | |
| A549 | Function Assay | 0.1mM | 24h | suppresses SU11274-induced cell death | 22466960 | |
| pDCs | Function Assay | 10mM | 0.5h | reduces the induction of IFN-α by ssRNA40 | 22396599 | |
| HeLa | Apoptosis Assay | 5mM | 24h | induces caspase-dependent cell death | 22545128 | |
| U251 | Apoptosis Assay | 5mM | 24h | increases S1-induced cell death | 22579788 | |
| MCF-7 | Apoptosis Assay | 0.1mM | 6h | enhances sirtinol-induced apoptosis | 22751989 | |
| PC-3 | Apoptosis Assay | 2mM | 2h | increases ORI-induced cell death | 22745580 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | DMSO | enhances apigenin-induced cell death | 24626522 |
| U2OS | Growth Inhibition Assay | 10mM | 24h | intensifies the growth inhibition induced by Dox | 24639013 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| HepG2 | Function Assay | 5mM | 4h | increases cellular levels of HL | 24713587 | |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| MDA-MB 231 | Apoptosis Assay | 5mM | 0.5h | modulates Tocomin® induced apoptosis | 24830781 | |
| PANC-1 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDA-MB-231 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MDA-MB-231 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| MCF-7 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MCF-7 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| HepG2 | Apoptosis Assay | 3mM | 5h | reduces cell apoptosis induced by QDs | 22836595 | |
| HeLa | Apoptosis Assay | 10mM | 2h | decreases cell viability co-treatment with PEI | 23000135 | |
| SK-HEP-1 | Apoptosis Assay | 10mM | 1h | protects against autophagy and induces apoptosis in bufalin-treated cells | 22858649 | |
| MDA-MB231 | Function Assay | 5mM | 1h | increases resveratrol-mediated caspase activation and cell death | 23088850 | |
| PaCa44 | Apoptosis Assay | 2.5mM | 1h | reduces genipin-mediated apoptosis | 23124112 | |
| T-47D | Function Assay | 10mM | 2h | inhibits autophagy process and increases rapamycin induced apoptosis | 23300026 | |
| GTL-16 | Apoptosis Assay | 5mM | 24h | reduces cell viability as compared to cells treated with MET inhibitors | 23313490 | |
| U251MG | Function Assay | 3mM | 1h | suppresses LC3-II protein expression | 23338618 | |
| T24 | Function Assay | 10mM | 1h | reduces the cleavage of LC3 after baicalin treatment | 23354080 | |
| HUVECs | Function Assay | 3mM | 24h | blocks the protective effect of resveratrol by inhibiting autophagy | 23358928 | |
| MCF-7 | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| Hela | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| OR6 | Function Assay | 10mM | 72h | suppresses HCV replication and formation of autophagosomes | 23395875 | |
| HT-29 | Function Assay | 1mM | 48/96h | inhibits AMPK induces autophagic cell death | 23508272 | |
| SH-SY5Y | Cytotoxicity Assay | 5mM | 24h | increases PCN toxicity | 23525265 | |
| Saos-2 | Apoptosis Assay | 1mM | 96h | increases cell death induced by PCX | 23563171 | |
| 1321N1 | Cytotoxicity Assay | 5mM | 24h | protects cell against PCN-induced toxicity | 23525265 | |
| A2780 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| OV2008 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| PC12 | Function Assay | 10mM | 24h | water | inhibits chymotrypsin-like proteasomal activity. | 23603979 |
| SH-SY5Y | Apoptosis Assay | 5mM | 1h | abolishes celastrol neuroprotective effect | 23619395 | |
| SH-SY5Y | Function Assay | 1mM | 24h | inhibits the autophagy induced by TOCP | 23743148 | |
| HepG2 | Function Assay | 10mM | 24h | inhibits siTIGAR- and HBSS-induced autophagy | 23817040 | |
| HeLa | Function Assay | 10mM | 2h | suppresses LC3 II expressison | 23864738 | |
| HONE-1 | Function Assay | 5mM | 1h | represses 6r-mediated ROS production | 23892358 | |
| MCF7 | Function Assay | 5mM | 24h | increases CuO induced cell death | 23962629 | |
| HO8910 | Apoptosis Assay | 10mM | 12h | enhances B19-induced apoptosi | 23983610 | |
| SMMC-7721 | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| Hep3B | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| H460 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| A549 | Function Assay | 10mM | 4h | inhibits autophagy induced by irradiation | 24142735 | |
| H1299 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| WiDr | Function Assay | 10mM | 1h | inhibits PCBL-induced LC3 II expression | 24190489 | |
| LoVo | Apoptosis Assay | 5mM | 48h | enhances DCA-induced apoptosis. | 24201812 | |
| HepG2 E47 | Function Assay | 2.5mM | 48h | increases the toxicity of AA, BSO, and CCl4 | 24273738 | |
| RKO | Function Assay | 2mM | 1h | DMSO | enhances cell death by geldanamycin | 24291777 |
| Hep3B | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| ACHN-5968 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| Huh7 | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| UOK257 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| ECSCs | Apoptosis Assay | 10mM | 4h | decreases rapamycin-treated apoptosis | 24319109 | |
| MCF-7 | Function Assay | 10mM | 24h | inhibits the autophagy induced by chemotherapy drugs | 24315578 | |
| SGC-7901 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| SMMC-7721 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| T24 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| NTUB1 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| MG-63 | Apoptosis Assay | 10mM | 12h | enhances DP-induced apoptosis | 24358301 | |
| MG-63 | Apoptosis Assay | 0.5/1mM | 32h | enhances salinomycin-induced cell apoptosis | 24358342 | |
| MG-63 | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| U2OS | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| HGC-27 | Function Assay | 10mM | 1h | inhibits the cell viability loss by RAD001 or MK-2206 | 24416349 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | enhances the apoptosis induced by apigenin | 24626522 | |
| A549 | Apoptosis Assay | 10mM | 48h | accelerates the reduction of cell viability induced by PTX | 24626722 | |
| Saos-2 | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| U2OS | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| HepG2 | Function Assay | 5mM | 4h | increases HL release | 24713587 | |
| A549 | Apoptosis Assay | 5mM | 48h | decreases the percentage of cell death and expression levels of caspase-3, Beclin-1 and LC3-II | 24706303 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| HT-29 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDR | Apoptosis Assay | 10mM | 6h | strengthens the power of anticancer drugs | 25019701 | |
| H157 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A549 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A2780cp | Growth Inhibition Assay | 1mM | 1h | increases cisplatin-induced cell death | 25322694 | |
| NBL-W-S | Apoptosis Assay | 1mM | 6h | increases cell apoptosis induced by GANT-61 | 25323222 | |
| NBL-W-S | Growth Inhibition Assay | 1mM | 6h | enhances GANT-61 toxicity | 25323222 | |
| A549 | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| 95D | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| A549 | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| 95D | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| Nara-H | Growth Inhibition Assay | 5mM | 48h | enhances temsirolimusmediated suppression of Nara-H cell proliferation | 21805033 | |
| HUVECs | Function Assay | 10mM | 0.5h | decreases the AGE-BSAinduced autophagy leve | 21468592 | |
| HepG2 | Apoptosis Assay | 2mM | 1h | enhances radiation-induced cell death | 21453691 | |
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 10 mg/mL
(67.04 mM)
Warmed with 50°C water bath;
Ultrasonicated;
Ethanol : 10 mg/mL Water : 4 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 149.15 | Formula | C6H7N5 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 5142-23-4 | Download SDF | Storage of Stock Solutions | Solutions are unstable. Prepare fresh or purchase small, pre-packaged sizes. Repackage upon receipt. | |
| Synonyms | NSC 66389 | Smiles | CN1C=NC(=N)C2=C1N=CN2 | ||
Mechanism of Action
| Targets/IC50/Ki |
Autophagy
Vps34
(HeLa cells) 25 μM
PI3Kγ
(HeLa cells) 60 μM
|
|---|---|
| In vitro |
The slight preference for Vps34 prevention by 3-Methyladenine (3-MA) probably arises from a hydrophobic ring specific to Vps34, which encircles the 3-methyl group of this compound. It has been reported to cause cancer cell death under both normal and starvation conditions, and could also suppress cell migration and invasion independently of its ability to inhibit autophagy, implying that it possesses functions other than autophagy suppression. This compound elicits caspase-dependent cell death that is independent of autophagy inhibition. Treatment with 5 mM of it reduces the percentage of glucose-starved HeLa cells displaying GFP-LC3 puncta to 23%. The levels of LC3-I are increasing and the levels of LC3-II are decreasing between 12 and 48 hours in cells that are treated with 3-MA. Conversion of LC3-I to LC3-II is suppressed by the compound. Treatment of HeLa cells with it at 2.5 mM or 5 mM for one day does not affect cell viability, whereas treatment with 10 mM for one day causes a 25.0% decrease in cell viability. Treatment of cells with 2.5, 5 or 10 mM for two days causes 11.5%, 38.0% and 79.4% decrease in viability, respectively. It decreases cell viability in a time- and dose-dependent manner, and significantly shortens the duration of nocodazole-induced-prometaphase arrest. Suppression of autophagy by 3-MA inhibits SU11274-induced cell death. Prolonged treatment with it (up to 9 hours) induces significant LC3 I to II conversion in wild type MEFs. Prolonged treatment with 3-MA, but not wortmannin, markedly increases GFP-LC3 punctuation/aggregation. Its induced LC3 conversion and free GFP liberation are ATG7-dependent. Treatment with it leads to evident increase of p62 protein level. The compound increases the p62 level even in Atg5−/− MEFs as well as in cells with DOX-mediated deletion of ATG5. It inhibits class I and class III PI3K in different temporal patterns. Its induced LC3 I to LC3 II conversion is dramatically compromised in Tsc2−/− cells compared with wild type cells. This compound disrupts the anti-autophagic function of mTOR complex 1. |
| Kinase Assay |
Protein degradation assay
|
|
HeLa cells are radiolabeled for 24 hours with 0.05 mCi/mL l-[U- 14C]valine. At the end of the labeling period, cells are rinsed three times with PBS. Cells are incubated for the designated times in either full medium or EBSS with or without the presence of 10 mM 3-Methyladenine (3-MA).
|
|
| In vivo |
3-Methyladenine (3-MA) blocks autophagy through its effect on class III phosphatidylinositol 3-kinase (PI3K). Treatment with this compound does not alter the degree of hemorrhage compared with the subarachnoid hemorrhage (SAH) group. Its pretreatment significantly aggravates neurological symptoms when compared with the SAH + vehicle group. Autophagy is decreased when it is applied. Conversely, cleaved caspase-3 is markedly up-regulated in the SAH + 3-MA group. In line with the up-regulation of cleaved caspase-3 expression, the number of TUNEL-positive cells in the right cortex is significantly increased in the SAH + 3-MA group compared with the SAH + vehicle group. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
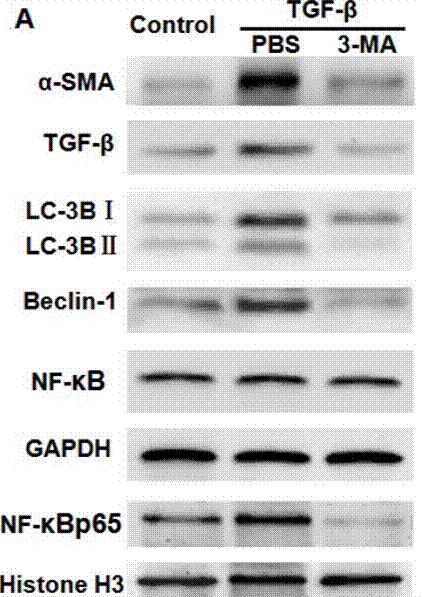
| Western blot | α-SMA / TGF-β / LC-3BI / LC-3B II / Beclin-1 / NF-κB p65 caspase-3 / caspase-9 / PARP VEGF APP / BACE1 / ADAM17 / Presenilin 1 / Presenilin 2 / Nicastrin / APH-1 / Pen-2 / LC3-1 / LC3-2 |
|
29296191 |
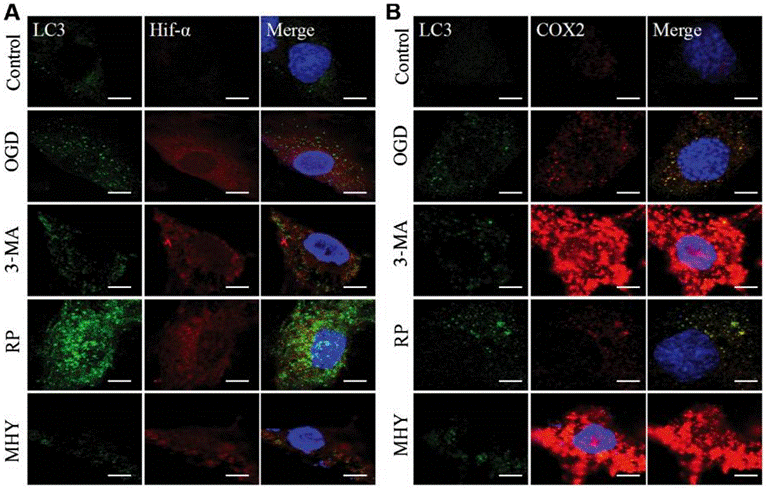
| Immunofluorescence | LC3 / Hif-α / COX2 |
|
29039446 |
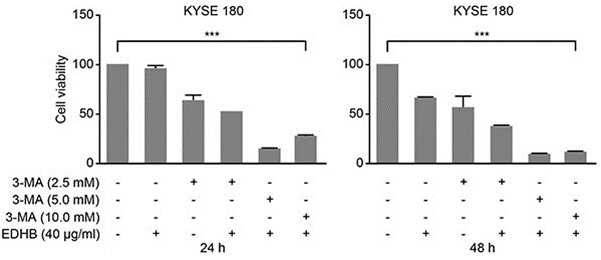
| Growth inhibition assay | Cell viability |
|
26934124 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
I'm also wondering whether it can be dissolved in water, or maybe something like culture medium, normal saline solution to form 10mM solution.
Answer:
As the reference (http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal. pone.0035665), it was found to inhibit autophagy at concentrations ranging from 1 to 10 mM and was directly dissolved into the culture medium at the indicated concentrations. And we tested the solubility of S2767, and found its solubility in DMEM is 31 mg/mL at about 40°C.






































