|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C6H7N5 |
||||||
| Molecular Weight | 149.15 | CAS No. | 5142-23-4 | ||||
| Solubility (25°C)* | In vitro | Ethanol | 6 mg/mL (40.22 mM) | ||||
| DMSO | 5 mg/mL (33.52 mM) | ||||||
| Water | 3 mg/mL (20.11 mM) | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | 3-Methyladenine (3-MA) is a selective PI3K inhibitor for Vps34 and PI3Kγ with IC50 of 25 μM and 60 μM in HeLa cells. It blocks class I PI3K consistently, whereas suppression of class III PI3K is transient, and also inhibits autophagosome formation. Solutions are unstable and should be fresh-prepared. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
||||||
| In vitro | The slight preference for Vps34 prevention by 3-Methyladenine (3-MA) probably arises from a hydrophobic ring specific to Vps34, which encircles the 3-methyl group of this compound. It has been reported to cause cancer cell death under both normal and starvation conditions, and could also suppress cell migration and invasion independently of its ability to inhibit autophagy, implying that it possesses functions other than autophagy suppression. This compound elicits caspase-dependent cell death that is independent of autophagy inhibition. Treatment with 5 mM of it reduces the percentage of glucose-starved HeLa cells displaying GFP-LC3 puncta to 23%. The levels of LC3-I are increasing and the levels of LC3-II are decreasing between 12 and 48 hours in cells that are treated with 3-MA. Conversion of LC3-I to LC3-II is suppressed by the compound. Treatment of HeLa cells with it at 2.5 mM or 5 mM for one day does not affect cell viability, whereas treatment with 10 mM for one day causes a 25.0% decrease in cell viability. Treatment of cells with 2.5, 5 or 10 mM for two days causes 11.5%, 38.0% and 79.4% decrease in viability, respectively. It decreases cell viability in a time- and dose-dependent manner, and significantly shortens the duration of nocodazole-induced-prometaphase arrest. Suppression of autophagy by 3-MA inhibits SU11274-induced cell death. Prolonged treatment with it (up to 9 hours) induces significant LC3 I to II conversion in wild type MEFs. Prolonged treatment with 3-MA, but not wortmannin, markedly increases GFP-LC3 punctuation/aggregation. Its induced LC3 conversion and free GFP liberation are ATG7-dependent. Treatment with it leads to evident increase of p62 protein level. The compound increases the p62 level even in Atg5−/− MEFs as well as in cells with DOX-mediated deletion of ATG5. It inhibits class I and class III PI3K in different temporal patterns. Its induced LC3 I to LC3 II conversion is dramatically compromised in Tsc2−/− cells compared with wild type cells. This compound disrupts the anti-autophagic function of mTOR complex 1. |
||||||
| In vivo | 3-Methyladenine (3-MA) blocks autophagy through its effect on class III phosphatidylinositol 3-kinase (PI3K). Treatment with this compound does not alter the degree of hemorrhage compared with the subarachnoid hemorrhage (SAH) group. Its pretreatment significantly aggravates neurological symptoms when compared with the SAH + vehicle group. Autophagy is decreased when it is applied. Conversely, cleaved caspase-3 is markedly up-regulated in the SAH + 3-MA group. In line with the up-regulation of cleaved caspase-3 expression, the number of TUNEL-positive cells in the right cortex is significantly increased in the SAH + 3-MA group compared with the SAH + vehicle group. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
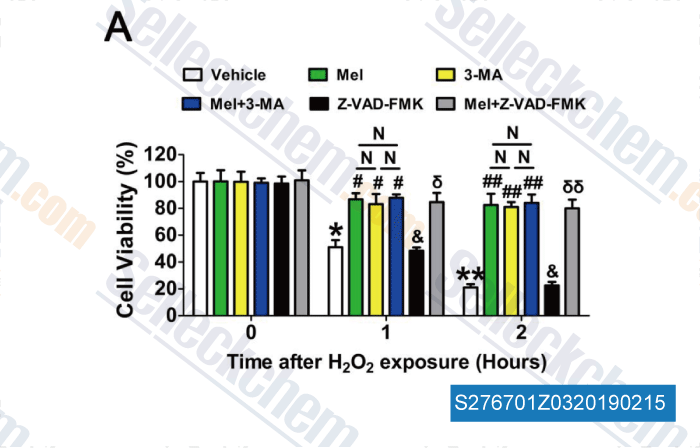
-
Data from [ , , Redox Biol, 2018, 18:138-157 ]
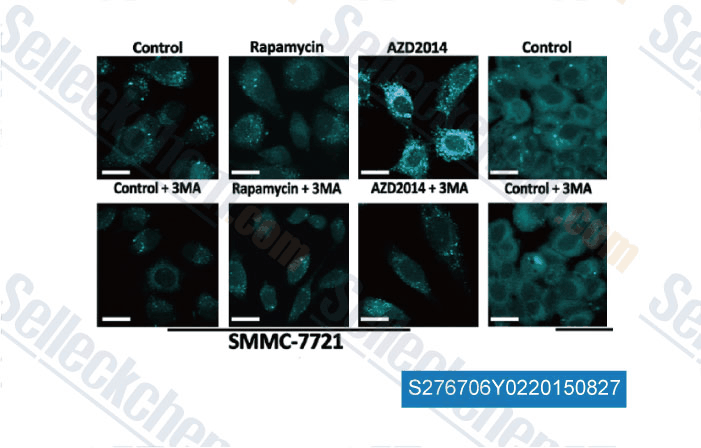
-
Data from [ , , Am J Cancer Res, 2015, 5(1): 125-139 ]
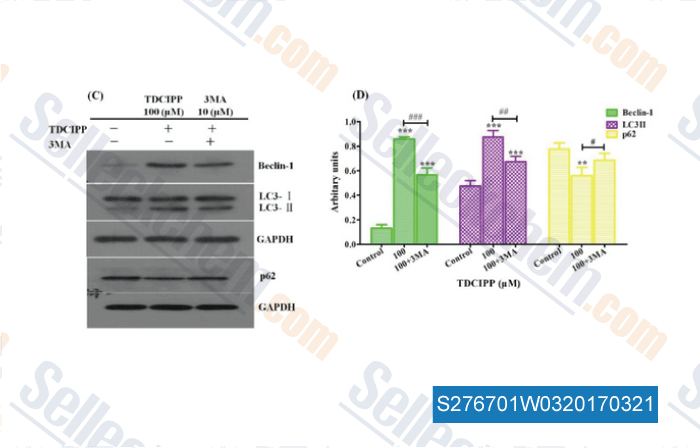
-
Data from [ , , Food Chem Toxicol, 2017, 100:183-196 ]
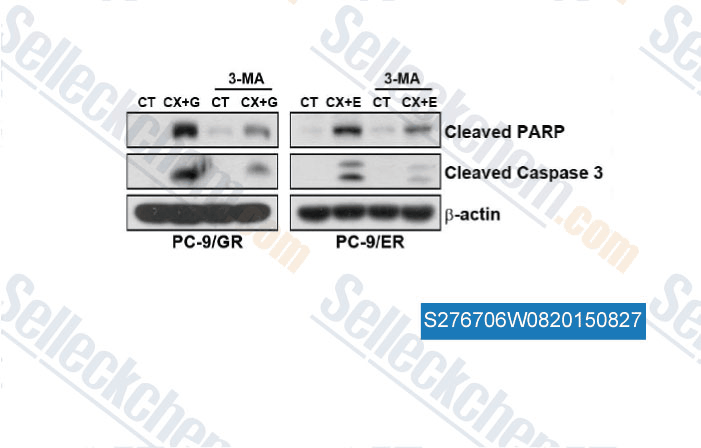
-
Data from [ , , PLoS One, 2014, 9(12): e114000 ]
Selleck's 3-Methyladenine (3-MA) Has Been Cited by 978 Publications
| Cooperative nutrient scavenging is an evolutionary advantage in cancer [ Nature, 2025, 10.1038/s41586-025-08588-w] | PubMed: 39972131 |
| Host glutathione is required for Rickettsia parkeri cell division and intracellular survival [ Nat Commun, 2025, 16(1):5547] | PubMed: 40593553 |
| Viruses hijack FPN1 to disrupt iron withholding and suppress host defense [ Nat Commun, 2025, 16(1):5912] | PubMed: 40595467 |
| RNF167 mediates atypical ubiquitylation and degradation of RLRs via two distinct proteolytic pathways [ Nat Commun, 2025, 16(1):1920] | PubMed: 39994288 |
| Unfolded protein response kinase PERK supports survival and metastasis of circulating tumor cell clusters via SAM synthesis and H3K4me3-dependent PDGFB signaling [ Cancer Commun (Lond), 2025, 45(12):1706-1733.] | PubMed: 41212905 |
| Extracellular LCN2 Binding to 24p3R in Astrocytes Impedes α-Synuclein Endocytosis in Parkinson's Disease [ Adv Sci (Weinh), 2025, 12(39):e01694] | PubMed: 40686313 |
| USP10 Inhibits Ferroptosis via Deubiquinating POLR2A in Head and Neck Squamous Cell Carcinoma [ Adv Sci (Weinh), 2025, 12(36):e12271] | PubMed: 40605431 |
| Disrupting AGR2/IGF1 paracrine and reciprocal signaling for pancreatic cancer therapy [ Cell Rep Med, 2025, 6(2):101927] | PubMed: 39914384 |
| TBK1 is a signaling hub in coordinating stress-adaptive mechanisms in head and neck cancer progression [ Autophagy, 2025, 1-23.] | PubMed: 40114316 |
| H3K36me2 methyltransferase NSD2/WHSC1 promotes triple-negative breast cancer metastasis via activation of ULK1-dependent autophagy [ Autophagy, 2025, 21(8):1824-1842] | PubMed: 40097917 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
