- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Sildenafil Citrate PDE inhibitor
Cat.No.S1431
Chemical Structure
Molecular Weight: 666.7
Jump to
Quality Control
Batch:
Purity:
99.83%
99.83
Solubility
|
In vitro |
DMSO
: 20 mg/mL
(29.99 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 666.7 | Formula | C22H30N6O4S.C6H8O7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 171599-83-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | UK-92480 Citrate | Smiles | CCCC1=NN(C2=C1N=C(NC2=O)C3=C(C=CC(=C3)S(=O)(=O)N4CCN(CC4)C)OCC)C.C(C(=O)O)C(CC(=O)O)(C(=O)O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
PDE5
(Cell-free assay) 3.5 nM
PDE6
(Cell-free assay) 33 nM
|
|---|---|
| In vitro |
Sildenafil citrate is a potent PDE type 5 reversible and selective inhibitor that blocks cGMP hydrolysis effectively (Ki ∼3 nM). Sildenafil exhibited high affinity for PDE type 5 and type 6 with inhibition constants (Ki) of ∼3.5 and 33 nM, respectively. Sildenafil enhances sodium nitroprusside- or transmural electrical stimulation-induced relaxation of precontracted corpus cavernosum muscle strips in organ baths, suggesting that sildenafil augments the activity of NO-mediated relaxation. Sildenafil citrate increases intracellular cGMP concentrations in cultured smooth muscle cells treated with sodium nitroprusside and in rabbit corpus cavernosum in vitro. Sildenafil is metabolized in the liver by cytochrome P450 and is converted into an active metabolite with characteristics similar to the parent compound.
|
| In vivo |
Sildenafil citrate enhances erectile function following pelvic nerve stimulation in anesthetized dogs as measured by increased intracavernosal pressure. Sildenafil citrate significantly reverses impaired carbachol-stimulated relaxation and inhibits superoxide formation by cavernosal tissue from hypercholesterolaemic rabbits. Sildenafil improves erectile function in a time- and dose-dependent fashion with maximization of erectile function recovery occurring with daily 20 mg/kg at the 28-day time point in Sprague-Dawley rats. Sildenafil use results in smooth muscle-collagen ratio protection and CD31 and eNOS expression preservation in Sprague-Dawley rats. Sildenafil reduces apoptotic indices significantly compared with control, and increases phosphorylation of akt and eNOS in Sprague-Dawley rats.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
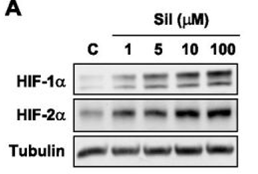
| Western blot | HIF-1α / HIF-2α VEGF / PDK1 / CA9 p-AKT / AKT / p-mTOR / mTOR |
|
23065129 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03686813 | Completed | Edema Pulmonary|Immersion|Diving |
Duke University|United States Department of Defense |
July 15 2019 | Phase 2 |
| NCT03417492 | Terminated | Traumatic Brain Injury|Mild Traumatic Brain Injury|Post-Concussion Syndrome |
University of Pennsylvania|Boston University |
March 1 2018 | Phase 1 |
| NCT03177824 | Unknown status | Fetal Growth Restriction |
Ain Shams University |
March 30 2017 | Phase 3 |
| NCT03262961 | Unknown status | Pre-Eclampsia; Mild |
Assiut University |
September 15 2016 | Phase 2|Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































