- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly FLAG Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Mifepristone
Synonyms: RU486, C-1073, RU 38486, RU-486
Mifepristone is a remarkably active antagonist of progesterone receptor and glucocorticoid receptor with IC50 of 0.2 nM and 2.6 nM, respectively. Mifepristone promotes cell autophagy and apoptosis, decreases Bcl-2 level and increases Beclin1 level, accompanied by weakened interaction between Bcl-2 and Beclin1.
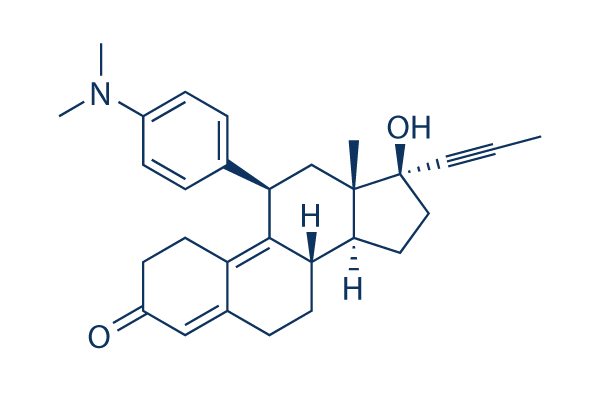
Mifepristone Chemical Structure
CAS: 84371-65-3
Selleck's Mifepristone has been cited by 27 Publications
1 Customer Review
Purity & Quality Control
Batch:
Purity:
100.00%
100.00
Mifepristone Related Products
| Related Targets | Estrogen receptor Progesterone receptor ERR | Click to Expand |
|---|---|---|
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Bioactive Compound Library-I Exosome Secretion Related Compound Library Human Hormone Related Compound Library | Click to Expand |
Signaling Pathway
Choose Selective Estrogen/progestogen Receptor Inhibitors
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| CHO-K1 cells | Function assay | Inhibition of CHO-K1 cells expressing glucocorticoid receptor, IC50=8e-06 μM | 15456242 | |||
| T47D-C124 cells | Function assay | 24 h | Antagonist activity at progesterone receptor in human T47D-C124 cells transfected with luciferase gene linked to MMTV promoter assessed as inhibition of progesterone-induced luciferase transactivation activity after 24 hrs, IC50=2.1e-05 μM | 19216549 | ||
| neuroblastoma cells | Function assay | In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor, IC50=2.5e-05 μM | 11150172 | |||
| T47D cells | Function assay | 48 h | Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity after 48 hrs, IC50=4.5e-05 μM | 19216549 | ||
| CV-1 cells | Function assay | Antagonistic activity against human progesterone receptor B (hPR-B) in co-transfected CV-1 cells, IC50=0.00018 μM | 9484511 | |||
| HEK293 cells | Function assay | Antagonist activity against glucocorticoid receptor (unknown origin) expressed in HEK293 cells by GRE-dependent luciferase reporter gene assay, IC50=0.000298 μM | 26218343 | |||
| COS7 cells | Function assay | Antagonist activity at cloned glucocorticoid receptor-ligand binding domain expressed in african green monkey COS7 cells by GAL4 luciferase reporter assay, IC50=0.0006 μM | 18504132 | |||
| SW1353 cells | Function assay | Binding affinity to glucocorticoid receptor in SW1353 cells by whole-cell binding assay, Ki=0.00082 μM | 17822897 | |||
| A549 cells | Function assay | Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luciferase reporter gene assay, IC50=0.0016 μM | 17317167 | |||
| A549 cells | Function assay | 16 h | Antagonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of corticoid-induced transcription after 16 hrs by glucocorticoid response element-driven luciferase reporter gene assay, IC50=0.0016 μM | 19217285 | ||
| rat H4-IIE cells | Function assay | 1 h | Antagonist activity against glucocorticoid receptor in rat H4-IIE cells assessed as inhibition of dexamethasone-induced receptor transactivation pre-incubated for 1 hr before dexamethasone addition and measured 24 hrs post dexamethasone stimulation by tyrosine aminotransferase enzyme assay, IC50=0.00194 μM | 26218343 | ||
| HeLa cells | Function assay | Effective concentration against inhibition of Dexamethasone induced glucocorticoid receptor transactivation of mouse mammary tumor virus luciferase gene in HeLa cells, EC50=0.002 μM | 16112571 | |||
| NIH3T3 cells | Function assay | In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptor, IC50=0.0022 μM | 11150172 | |||
| CHO cells | Function assay | Inhibition of Dexamethasone stimulated transcriptional activity in CHO cells expressing glucocorticoid receptor, IC50=0.005 μM | 12824023 | |||
| hGRAF cells | Function assay | Inhibition of human GR expressed in hGRAF cells, Ki=0.005 μM | 17070047 | |||
| COS-1 | Function assay | Binding affinity for human androgen receptor in transiently-transfected COS-1 cells, Ki=0.022 μM | 9484511 | |||
| rat hepatocytes | Function assay | Inhibition of dexamethasone-induced GR-mediated tyrosine amino transferase activity in rat hepatocytes, IC50=0.27 μM | 15261266 | |||
| human K562/R7 cells | Function assay | 72 h | Potentiation of doxorubicin-induced cytotoxicity against doxorubicin-resistant human K562/R7 cells assessed as doxorubicin IC50 at 1 uM after 72 hrs by MTT assay, IC50=0.9 μM | 25634041 | ||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Mifepristone is a remarkably active antagonist of progesterone receptor and glucocorticoid receptor with IC50 of 0.2 nM and 2.6 nM, respectively. Mifepristone promotes cell autophagy and apoptosis, decreases Bcl-2 level and increases Beclin1 level, accompanied by weakened interaction between Bcl-2 and Beclin1. | ||||||
|---|---|---|---|---|---|---|---|
| Features | Mifepristone is the first approved medication for patients with endogenous cushing | ||||||
| Targets |
|
| In vitro | ||||
| In vitro | Mifepristone inhibit corticoid-induced transcription from a glucocorticoid response element (GRE)-linked luciferase reporter gene in the human lung carcinoma cell line A549. Moreover, Mifepristone also blocks progesterone induction of alkaline phosphatase activity in the human breast cancer cell line T47D. [1] Mifepristone inhibits ovarian cancer cell growth of SK-OV-3 and OV2008 with IC50 of 6.25 μM and 6.91 μM, respectively. [2] A recent study shows that Mifepristone induces caspase-1 over expression both in differentiated and undifferentiated caspase-1-embryonic stem cells. [3] |
|||
|---|---|---|---|---|
| Kinase Assay | Glucocorticoid receptor (GR) antagonist activity, Progesterone receptor (PR) antagonist activity | |||
| T47D alkaline phosphatase assay: T47D human breast cancer cells are plated in 96-well tissue culture plates at 104 cells per well in assay medium [RPMI medium without phenol red containing 5% (v/v) charcoal-treated FBS and 1% (v/v) penicillin–streptomycin]. Two days later, the medium is decanted and Mifepristone or control is added at a final concentration of 0.1% (v/v) dimethylsulfoxide in fresh assay medium. Twenty-four hours later, an alkaline phosphatase assay is performed using a SEAP kit. The medium is decanted and the cells are fixed for 30 minutes at room temperature with 5% (v/v) formalin. The cells are washed once at room temperature with Hanks | ||||
| Cell Research | Cell lines | OV2008 and SK-OV-3 cells | ||
| Concentrations | 0-20 μM | |||
| Incubation Time | 24 hours | |||
| Method | Cell growth is evaluated in various ovarian cancer cell lines that are subjected to dose-response or time course treatments. Media containing each of the doses of fresh steroids is replaced every 24 hours. Control groups of cells are treated with vehicle ethanol at a final concentration of less than 0.05%. Number of viable cells is evaluated by trypsinization and counting in a hemocytometer chamber using trypan blue dye exclusion. Experiments are conducted in media without phenol red and supplemented with charcoal extracted fetal bovine serum, or media containing unextracted serum and having phenol red. Similar results are obtained with both media preparations; therefore, after performing the growth curves, all subsequent experiments are conducted using media with unextracted serum and in the presence of phenol red. When indicated, the proliferation IC50s are calculated using software designed to study drug interaction. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p-AKT / AKT / p-ERK / ERK MMP-2 / MMP-9 / COX-2 / VEGF |
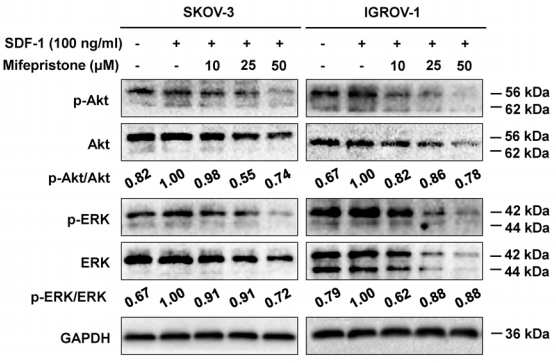
|
28938623 | |
| In Vivo | ||
| In vivo |
Mifepristone can impair the growth of SK-OV-3 tumors in immunosuppressed mice at 0.5 mg/day and 1 mg/day. [2] Mifepristone inhibits the prostate weight significantly in the highest doses in vivo, and inhibits growth of the prostate gland produced by dihydrotestosterone (DHT) to a greater extent than the induction of atrophy and cell death in rats. [4] |
|
|---|---|---|
| Animal Research | Animal Models | SK-OV-3 ovarian cancer cells are injected into immunosuppressed mice. |
| Dosages | 0.5 or 1 mg/day | |
| Administration | Implanted s.c. with pellets | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05177510 | Recruiting | Labor Induced |
Chelsea and Westminster NHS Foundation Trust |
August 25 2023 | Phase 3 |
| NCT04905251 | Recruiting | Medical Abortion |
Linepharma International LTD |
February 22 2022 | -- |
| NCT05062174 | Withdrawn | BRCA1 Mutation|High-grade Serous Ovarian Cancer|TNBC - Triple-Negative Breast Cancer |
Indiana University|Breast Cancer Research Foundation |
November 1 2021 | -- |
| NCT04588688 | Terminated | Central Adrenal Insufficiency|Mifepristone |
Tobias Else|Corcept Therapeutics|University of Michigan |
May 5 2021 | Phase 2 |
| NCT03659045 | Completed | Abortion-Related Disorders |
Assistance Publique Hopitaux De Marseille |
January 15 2019 | Not Applicable |
| NCT03258372 | Completed | Healthy |
Corcept Therapeutics |
August 16 2017 | Phase 1 |
Chemical Information & Solubility
| Molecular Weight | 429.59 | Formula | C29H35NO2 |
| CAS No. | 84371-65-3 | SDF | Download Mifepristone SDF |
| Smiles | CC#CC1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 85 mg/mL ( (197.86 mM); Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 85 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Mifepristone | Mifepristone ic50 | Mifepristone price | Mifepristone cost | Mifepristone solubility dmso | Mifepristone purchase | Mifepristone manufacturer | Mifepristone research buy | Mifepristone order | Mifepristone mouse | Mifepristone chemical structure | Mifepristone mw | Mifepristone molecular weight | Mifepristone datasheet | Mifepristone supplier | Mifepristone in vitro | Mifepristone cell line | Mifepristone concentration | Mifepristone nmr