research use only
Ketorolac COX inhibitor
Cat.No.S1646
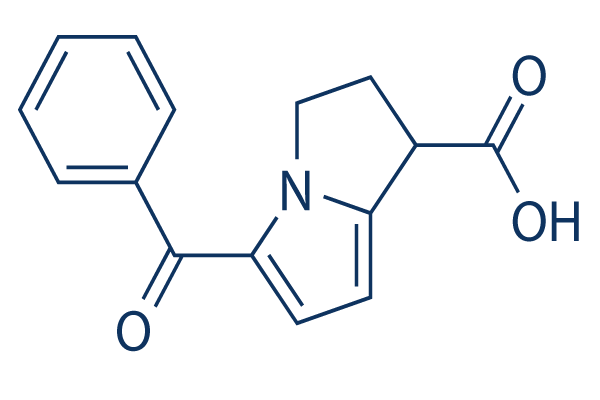
Chemical Structure
Molecular Weight: 255.27
Quality Control
Solubility
|
In vitro |
DMSO
: 51 mg/mL
(199.78 mM)
Ethanol : 51 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 255.27 | Formula | C15H13N1O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 74103-06-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CN2C(=CC=C2C(=O)C3=CC=CC=C3)C1C(=O)O | ||
Mechanism of Action
| Features |
A COX-1 preferential inhibitor among currently marked nonsteroidal anti-inflammatory drugs (NSAIDs).
|
|---|---|
| Targets/IC50/Ki |
COX-1 (human)
1.23 μM
COX-2 (human)
3.50 μM
|
| In vitro |
(R, S)-, (S)-, and (R)-Ketorolac inhibit both isoforms of COX in recombinant rat and human enzyme systems, and similar as inhibitors of rat COX (rCOX) and human COX (hCOX) under the conditions used. This compound inhibits rat COX-1, rat COX-2, human COX-1 and human COX-2 with IC50 of 0.27 μM, 2.06 μM, 1.23 μM and 3.50 μM, respectively. The (S) enantiomer of this chemical with IC50 of 0.10 μM for rat COX-1 is approximately twice as potent as the racemate, whereas the (R)-enantiomer with IC50 of > 100 μM is virtually without activity. It shows inhibition of eicosanoid formation in HEL cells (COX-1) and LPS-stimulated Mono Mac 6 cells (COX-2) with IC50 of 0.025 μM and 0.039 μM, respectively, but does not significantly inhibit NO accumulation in supernatants of LPS-stimulated RAW 264.7 cells up to 300 μM. This compound significantly inhibits thymidine incorporation of human osteoblasts (hOBs) upon 24 hours treatment in a dose-dependent manner, and inhibits proliferation and arrests cell cycle at G0/G1 phase in hOBs.
|
| Kinase Assay |
Inhibition of Prostaglandin Formation
|
|
Recombinant COX-1 and COX-2 from rat (rCOX) and human (hCOX) expressed in a baculovirus system are purified and reconstituted with 2 mM phenol and 1 μM hematin. Then the cyclooxygenase activity is measured using a radiometric assay, and the specific activity of the final enzyme preparations used is between 20,000 and 35,000 units. Ketorolac (2 -15 μL) are diluted in DMSO and preincubated with the appropriate recombinant COX (3 -15 ng) at a final concentration of 0.01 to 1000 μM in a reaction mixture (150 μL) containing 50 mM Tris-HCl buffer (pH 7.9), 2 mM EDTA, 10% glycerol, 2 mM phenol, and 1 μM hematin for 10 minutes. The reaction is initiated by addition of [14C]arachidonic acid (50–60 mCi/mmol in a final concentration of 20 μM) and is terminated 45 seconds later by the addition of 100 μL of 0.2 N HCl and 750 μL of distilled water. The total reaction volume is then applied to a 1 mL C18 Sep-pak column that has previously been washed with 2 mL of methanol followed by 5 mL of deionized water. Oxygenated products are eluted with 3 mL of a mixture of acetonitrile/water/acetic acid (50:50:0.1, v/v/v) and quantified by liquid scintillation spectroscopy.
|
|
| In vivo |
(R, S)-Ketorolac is significantly more potent than indomethacin or diclofenac sodium in tests of acetic acid-induced writhing, carrageenan-induced paw hyperalgesia, and carrageenan-induced edema formation in rats, with ID50 of 0.24, 0.29 and 0.08 mg/kg, respectively. This compound produces significant inhibition of COX-1 activity and gastric PG synthesis with doses of ≥1 mg/kg inhibiting COX-1 activity by 95% and gastric PG synthesis by >88%. It does not significantly affect COX-2 activity at doses of ≤3 mg/kg, but at doses of 10 and 30 mg/kg, it produces significant inhibition of COX-2 activity by 75% and 91%, respectively. This chemical causes gastric damage in rats only at doses that inhibits both COX-1 and COX-2, or when given with a COX-2 inhibitor.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05776953 | Recruiting | Renal Colic|Flank Pain|Emergencies|Analgesia |
Hackensack Meridian Health |
December 21 2023 | Phase 4 |
| NCT05336968 | Recruiting | Osteoarthritis Knee |
United Health Services Hospitals Inc. |
September 15 2022 | Phase 4 |
| NCT05336266 | Recruiting | Pancreas Cancer|Pancreatic Ductal Adenocarcinoma|Pancreatic Cancer |
Andrew Hendifar MD|Yinuoke Ltd.|Cedars-Sinai Medical Center |
July 1 2022 | Early Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































