Our Quality Standards
At Selleckchem, quality is not an optionâit is the foundation of our business. We understand that impure reagents can ruin months of research. That is why we employ a comprehensive quality control system that exceeds industry standards.
For every single batch of our products, we perform the following validation tests:
-
High-Performance Liquid Chromatography (HPLC): Used to verify purity. We ensure that our inhibitors meet the highest purity standards (typically >99%) to eliminate potential side effects caused by impurities.
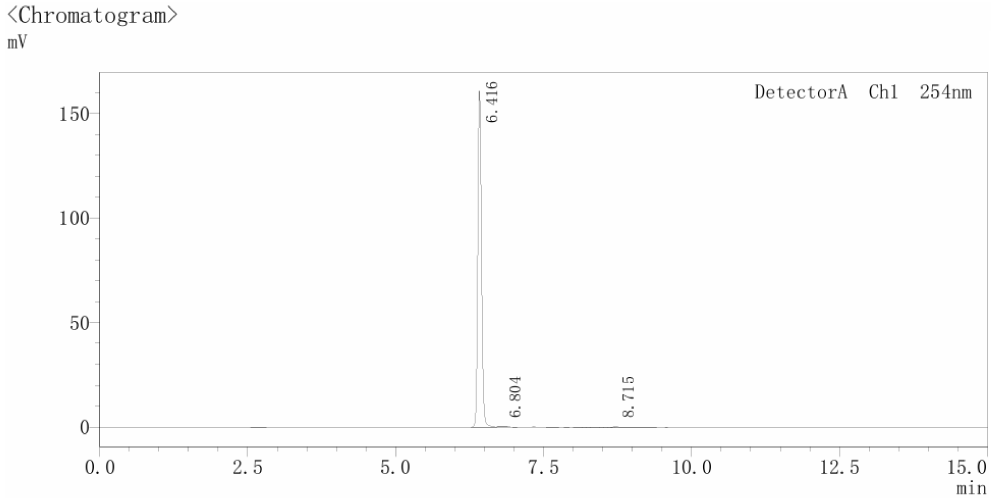
Figure 1. HPLC Chromatogram of S7963 (Lot 9).
To illustrate the tangible difference in quality that Selleckchem provides, we present a direct side-by-side HPLC comparison of our MGD-28 (Cat. No. E4724) against the same compound obtained from a leading competitor.
Comparative Analysis: Selleckchem MGD-28 (Cat. No. E4724) vs. Competitor
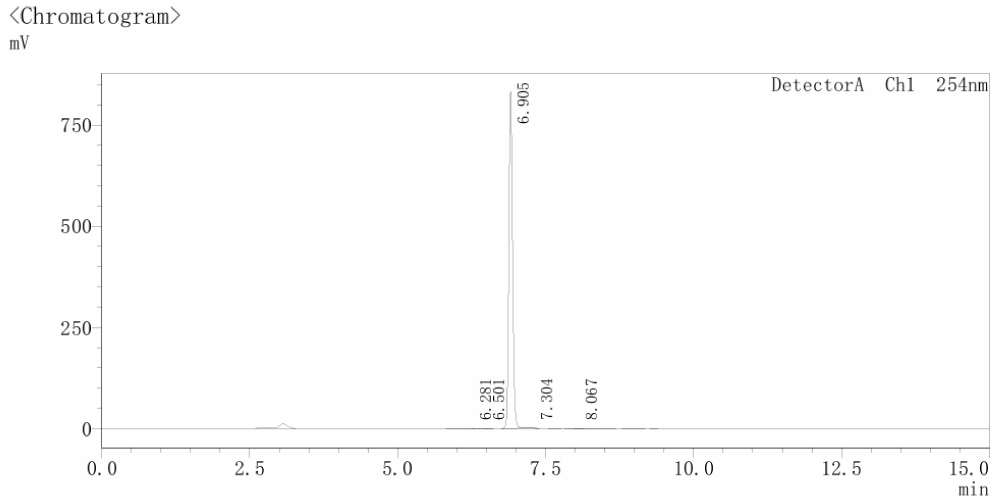
Figure 2. HPLC Chromatogram of Selleckchem MGD-28 (Cat. No. E4724).
This chromatogram demonstrates the superior purity of Selleckchem's E4724. The analysis reveals a clean baseline with a single, sharp dominant peak corresponding to the target compound. The absence of significant secondary peaks indicates a highly pure sample (>99%), ensuring that observed experimental biological activity is due solely to E4724.
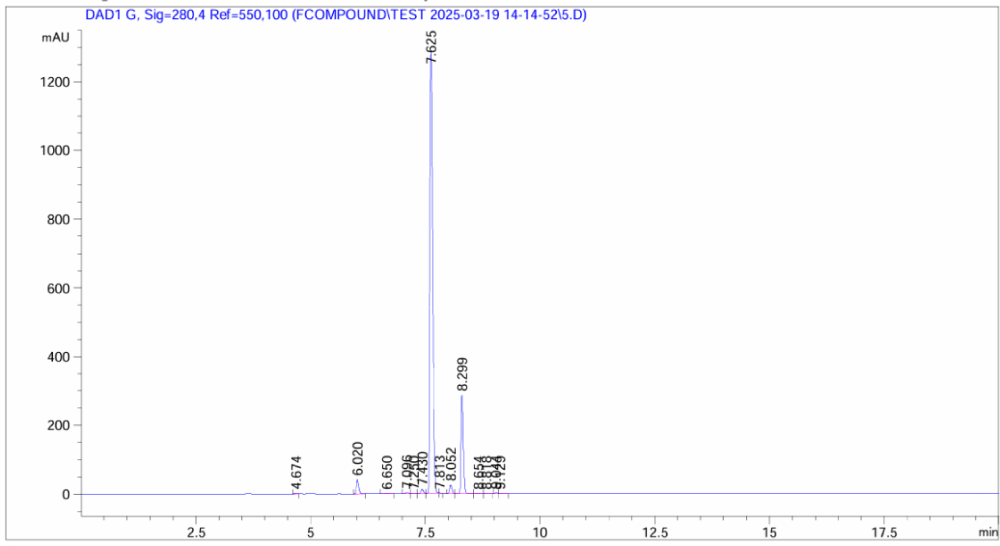
In sharp contrast, the HPLC analysis of MGD-28 purchased from a competitor shows a significantly lower quality profile. The chromatogram displays multiple distinct impurity peaks eluting before and after the main compound, along with a noisier baseline. These impurities constitute a significant percentage of the sample mass and pose a high risk of interfering with sensitive biological assays.
-
Nuclear Magnetic Resonance (NMR): Used to verify chemical structure. We perform 1H-NMR to confirm that the molecular structure matches the design perfectly.
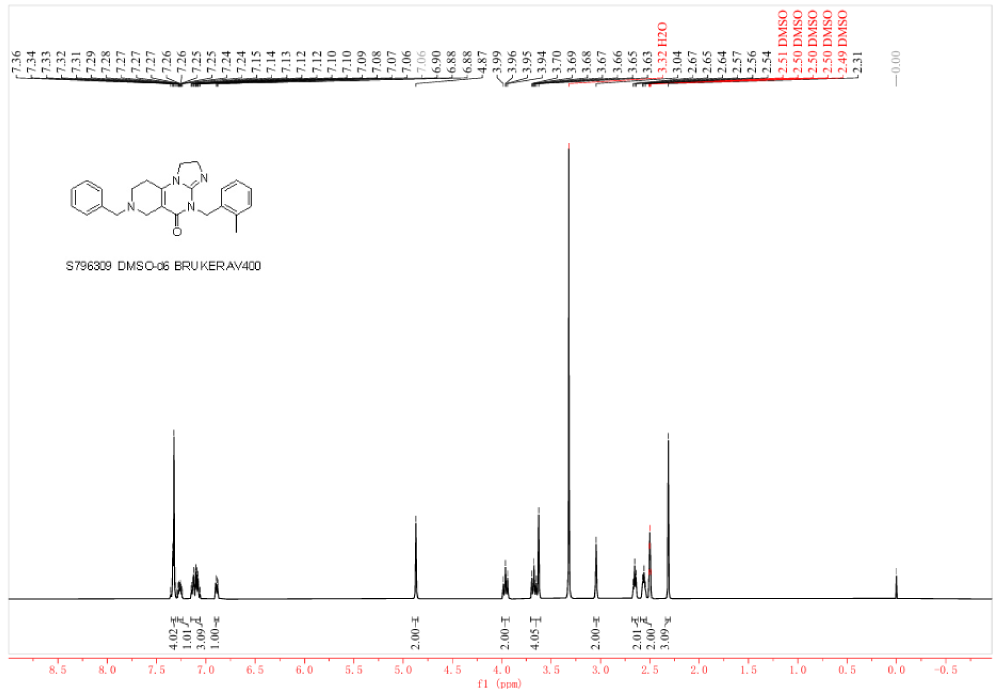
Figure 4. 1H NMR Spectrum of S7963 (Lot 9) in DMSO-d6.
- Microanalysis: Used to verify elemental composition. We perform elemental analysis (C, H, N) to confirm the chemical formula and ensure the absence of residual solvents or moisture.
Batch-to-Batch Consistency
Reproducibility is the crisis of modern science. To ensure your experiments are reproducible over time, we guarantee consistency across different production lots.
We compare the analytical data of every new batch against our "Gold Standard" reference to ensure no variation in purity, solubility, or biological activity.
- No surprise variations.
- No hidden impurities.
- Just reliable results, every time.
Transparency You Can Trust
We believe in total transparency. The quality control data for your specific product is always available.
- Downloadable COA: You can download the Certificate of Analysis (COA), HPLC, MS, and NMR spectra for your specific batch directly from our website.
- Real Data: We display actual spectral images, not generic placeholders.





































