research use only
umbralisib (TGR-1202) PI3K inhibitor
Cat.No.S8194
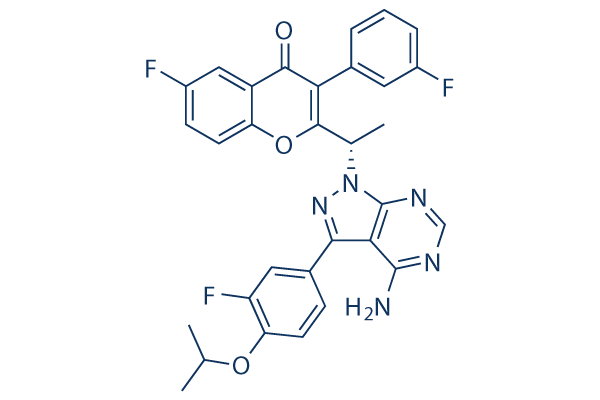
Chemical Structure
Molecular Weight: 571.55
Quality Control
| Related Targets | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other PI3K Inhibitors | LY294002 Buparlisib (BKM120) SAR405 Eganelisib (IPI-549) XL147 analogue Paxalisib (GDC-0084) Tersolisib (STX-478) 3-Methyladenine (3-MA) Dactolisib (BEZ235) Pictilisib (GDC-0941) |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(174.96 mM)
Ethanol : 6 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 571.55 | Formula | C31H24F3N5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1532533-67-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Rp-5264 | Smiles | CC(C)OC1=C(C=C(C=C1)C2=NN(C3=NC=NC(=C23)N)C(C)C4=C(C(=O)C5=C(O4)C=CC(=C5)F)C6=CC(=CC=C6)F)F | ||
Mechanism of Action
| Targets/IC50/Ki |
PI3Kδ
(Cell-free assay) 22.2 nM
|
|---|---|
| In vitro |
The compound displays a high degree of selectivity over the alpha (>1000 fold), beta (>30-50 fold), and gamma (>15-50 fold) isoforms. Additionally, the compound causes a half-maximal inhibition of human whole blood CD19 cell proliferation between 100-300 nM. Treatment of PBMC with RP5264 results initially in a G2/M arrest followed by subsequent increase in the number of Sub G0 cells. Viability assays demonstrate that the compound causes a significant inhibition in growth as well as Akt phosphorylation of immortalized and primary leukemic cells.
|
| In vivo |
The compound exhibits good oral absorption with favourable pharmacokinetic properties in rodents. It also has an excellent safety profile.
|
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03207256 | Terminated | Chronic Lymphocytic Leukemia|Non Hodgkin Lymphoma |
TG Therapeutics Inc. |
August 9 2017 | Phase 2 |
| NCT03178201 | Terminated | Follicular Lymphoma |
Columbia University|TG Therapeutics Inc. |
August 20 2017 | Phase 2 |
| NCT02867618 | Terminated | Hodgkin Disease|Lymphoma Non-hodgkin |
Columbia University |
October 16 2016 | Phase 1|Phase 2 |
| NCT02493530 | Active not recruiting | Myelofibrosis|Polycythemia Vera |
Vanderbilt-Ingram Cancer Center |
July 2015 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































