research use only
Radiprodil (RGH-896) NMDAR antagonist
Cat.No.S6644
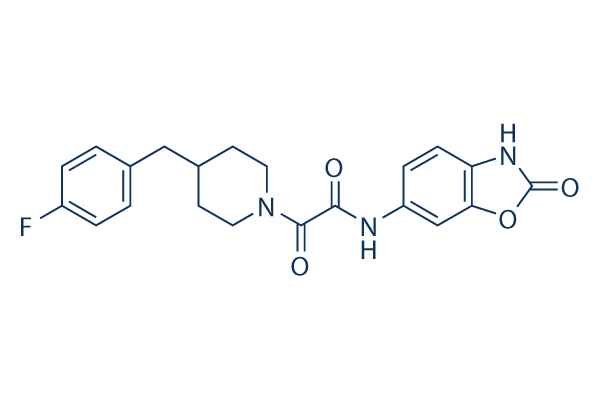
Chemical Structure
Molecular Weight: 397.40
Quality Control
Solubility
|
In vitro |
DMSO
: 79 mg/mL
(198.79 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 397.40 | Formula | C21H20FN3O4 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 496054-87-6 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CN(CCC1CC2=CC=C(C=C2)F)C(=O)C(=O)NC3=CC4=C(C=C3)NC(=O)O4 | ||
Mechanism of Action
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06392009 | Recruiting | Tuberous Sclerosis Complex|Focal Cortical Dysplasia |
GRIN Therapeutics Inc. |
May 2024 | Phase 1|Phase 2 |
| NCT05818943 | Recruiting | GRIN-related Disorders |
GRIN Therapeutics Inc. |
March 7 2023 | Phase 1 |
| NCT02829827 | Terminated | Infantile Spasms (IS) |
UCB Biopharma S.P.R.L.|UCB Pharma |
December 4 2017 | Phase 2 |
| NCT02647697 | Completed | Healthy Volunteers |
UCB Biopharma S.P.R.L.|PRA Health Sciences|UCB Pharma |
January 2016 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































