research use only
LXR-623 (WAY-252623) LXR Agonist
Cat.No.S8390
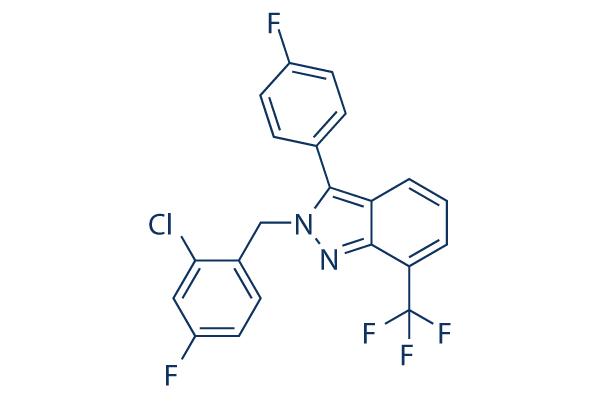
Chemical Structure
Molecular Weight: 422.78
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other Liver X Receptor Inhibitors | GW3965 Hydrochloride T0901317 GSK2033 SR9243 Abequolixron (RGX-104) AZ876 Desmosterol GSK3987 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| THP1 cells | Function assay | Effect on ABCA1 gene expression in human differentiated THP1 cells, EC50=0.54 μM | ||||
| HepG2 cells | Function assay | Effect on triglyceride accumulation in human HepG2 cells, EC50=1 μM | ||||
| HuH7 cells | Function assay | Agonist activity at human LXRbeta ligand binding domain expressed in human HuH7 cells co-transfected with Gal4-DBD by transactivation assay, EC50=3.67 μM | ||||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 84 mg/mL
(198.68 mM)
Ethanol : 84 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 422.78 | Formula | C21H12ClF5N2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 875787-07-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | WAY-252623 | Smiles | C1=CC2=C(N(N=C2C(=C1)C(F)(F)F)CC3=C(C=C(C=C3)F)Cl)C4=CC=C(C=C4)F | ||
Mechanism of Action
| Targets/IC50/Ki |
LXR-β
24 nM
LXR-α
179 nM
|
|---|---|
| In vitro |
LXR-623 suppresses LDLR expression, increases expression of the ABCA1 efflux transporter, and induces substantial cell death in all of the GBM samples tested. The brain metastatic breast cancer cell line MDA-MB-361, which harbors ERBB2 amplification, is also highly sensitive to this compound-dependent cell death in a concentration-dependent manner. It inhibits LDL uptake and induces cholesterol efflux in GBM cells, resulting in a significant reduction in cellular cholesterol content. Normal brain cell insensitivity to this chemical may be due to reliance on endogenous synthesis of cholesterol and intact negative feedback through synthesis of endogenous oxysterols.
|
| In vivo |
LXR-623 is absorbed rapidly with peak concentrations (Cmax) achieved at approximately 2 hours. The Cmax and area under the concentration-time curve increases in a dose-proportional manner. The mean terminal disposition half-life is between 41 and 43 hours independently of dose. In a low-density lipoprotein (LDL) receptor, (LDLr) knockout mouse model of atherosclerosis, this compound administered orally upregulates intestinal ABCG5 and ABCG8 and reduces atheroma burden without altering serum or hepatic cholesterol and trig-lycerides. It shows brain penetration and causes tumor regression in a GBM(glioblastomas) mouse model, reducing cholesterol and inducing cell death.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































