research use only
Formoterol Hemifumarate Adrenergic Receptor agonist
Cat.No.S2020
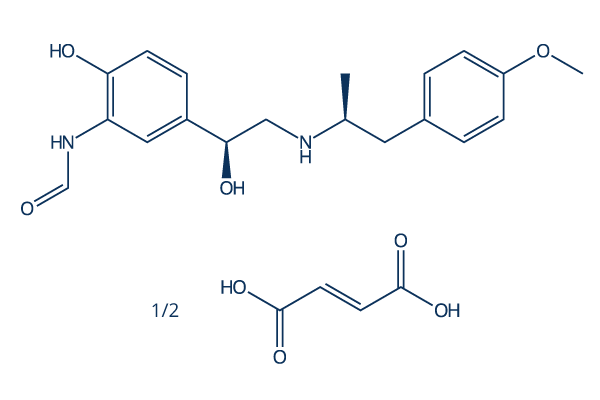
Chemical Structure
Molecular Weight: 402.40
Quality Control
| Related Targets | AChR 5-HT Receptor COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Adrenergic Receptor Inhibitors | ICI 118551 Hydrochloride (Zenidolol) L755507 Yohimbine HCl Atipamezole Higenamine hydrochloride Detomidine HCl Naftopidil Demethyl-Coclaurine Buflomedil HCl Fenoterol hydrobromide |
Solubility
|
In vitro |
DMSO
: 80 mg/mL
(198.8 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 402.40 | Formula | C19H24N2O4.1/2C4H4O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 43229-80-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Eformoterol, CGP 25827A, NSC 299587, YM 08316,Formoterol fumarate | Smiles | CC(CC1=CC=C(C=C1)OC)NCC(C2=CC(=C(C=C2)O)NC=O)O.CC(CC1=CC=C(C=C1)OC)NCC(C2=CC(=C(C=C2)O)NC=O)O.C(=CC(=O)O)C(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
β2-adrenergic receptor
|
|---|---|
| In vitro |
Formoterol is a potent airway smooth muscle relaxant with high efficacy, and very high affinity and selectivity for the beta 2-adrenoceptor. Formoterol appears to be retained in airway smooth muscle for extended periods since its relaxant effect on human airway smooth muscle is resistant to repeated washing and formoterol displays 'reassertion' of relaxation after washout of a beta-adrenoceptor antagonist. Formoterol has been demonstrated to potently inhibit these cells and processes in experimental test systems. Formoterol, like salbutamol and salmeterol, relaxes isolated preparations of guinea-pig trachea and human bronchus, and inhibits antigen-induced mediator release from human lung fragments in a concentration-related fashion. |
| In vivo |
Formoterol causes dose-related inhibition of histamine-induced bronchoconstriction in conscious guinea-pigs. Formoterol inhibits histamine-induced plasma protein extravasation (PPE) in guinea-pig lung, with significant inhibition being observed at 10 mg /mL and 100 mg /mL. Formoterol (100 mg /mL) inhibits PPE in guinea-pig lung for 2-4 hours, a duration of action intermediate between that previously obtained for salbutamol (1 hour) and salmeterol (> 6 hours). Formoterol inhibits neutrophil accumulation (lipopolysaccharide-induced) in guinea-pig lung but at doses greater than those required to inhibit granulocyte-independent PPE (histamine-induced). Formoterol (100 mg /mL) inhibits PAF-induced eosinophil accumulation in guinea-pig lung. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06102005 | Recruiting | Asthma |
Sanofi |
October 16 2023 | Phase 2 |
| NCT05421598 | Active not recruiting | Asthma |
Sanofi |
June 30 2022 | Phase 2 |
| NCT04663386 | Terminated | Asthma|COPD |
Orion Corporation Orion Pharma |
December 10 2020 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































