research use only
Cefoxitin Bacterial chemical
Cat.No.S5951
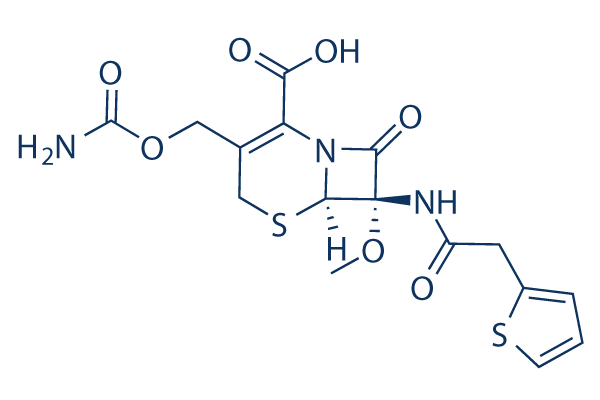
Chemical Structure
Molecular Weight: 427.45
Quality Control
| Related Targets | Integrase Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Bacterial Inhibitors | Berberine BTZ043 Racemate Teicoplanin Pefloxacin Mesylate Furagin Ornidazole Proanthocyanidins Solithromycin Skatole Berberine Sulfate |
Solubility
|
In vitro |
DMSO
: 85 mg/mL
(198.85 mM)
Ethanol : 2 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 427.45 | Formula | C16H17N3O7S2 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 35607-66-0 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | COC1(C2N(C1=O)C(=C(CS2)COC(=O)N)C(=O)O)NC(=O)CC3=CC=CS3 | ||
Mechanism of Action
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04775238 | Unknown status | Nosocomial Infections |
Sohag University |
February 27 2021 | Not Applicable |
| NCT03269994 | Active not recruiting | Pancreatic Cancer|Pancreas Cancer|Pancreatic Diseases |
Memorial Sloan Kettering Cancer Center|Indiana University|Massachusetts General Hospital|Thomas Jefferson University|Washington University School of Medicine|Advocate Illinois Masonic Medical Center|Baptist Memorial Health Care Corporation|Baylor Scott and White Health|The Cleveland Clinic|Emory University|Fox Chase Cancer Center|Gundersen Lutheran Medical Center|Hackensack Meridian Health|Hamilton Health Sciences Center|Intermountain Health Care Inc.|Jersey Shore Medical Center (Hackensack Meridian)|Johns Hopkins University|Montefiore Medical Center/Albert Einstein College of Medicine|North Shore University HealthSystem|Milton S. Hershey Medical Center|Rhode Island Hospital|Stony Brook Medicine|Sunnybrook Health Sciences Centre Canada|Temple University|The Ohio State University Wexner Medical Center|The Ottowa Hospital/University of Ottowa|University of California Davis|University of Chicago|University of Iowa|University of Texas Southwestern Medical Center|University of Utah|University of Wisconsin Madison|Providence Health & Services|Albany Medical College|Northwestern University|Universtiy of Mississippi Medical Center|Mount Sinai Hospital New York|Brody School of Medicine at East Carolina University |
November 21 2017 | Phase 3 |
| NCT03306290 | Completed | Obese|Antibiotic Prophylaxis|Bariatric Surgery Candidate |
Central Hospital Nancy France |
October 30 2017 | Not Applicable |
| NCT02703857 | Completed | Antibiotic Prophylaxis Surgery |
Poitiers University Hospital |
February 2016 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































