research use only
6AN (6-Aminonicotinamide) Dehydrogenase inhibitor
Cat.No.S9783
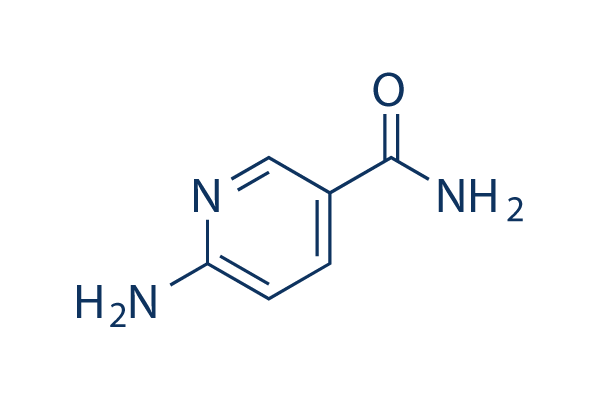
Chemical Structure
Molecular Weight: 137.14
Quality Control
Solubility
|
In vitro |
DMSO
: 27 mg/mL
(196.87 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 137.14 | Formula | C6H7N3O |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 329-89-5 | -- | Storage of Stock Solutions |
|
|
Mechanism of Action
| Targets/IC50/Ki |
G6PD
(Cell-free assay) 0.46 μM(Ki)
|
|---|---|
| In vitro |
Blocking the pentose phosphate pathway (PPP) with 6-AN (6-Aminonicotinamide) significantly inhibits mES colony formation in the presence of a normal concentration of glucose. This compound effectively inhibits AKT phosphorylation and activation of its downstream effector S6, while having no effect on the ERK and PDK1 activities, suggesting an AKT-specific effect. It also inhibits cell growth. |
| In vivo |
PTEN null human cancer cells and in vivo murine models are sensitive to 6-AN (6-Aminonicotinamide, anti-PPP, anti pentose phosphate pathway) treatments, suggesting the importance of the PPP in maintaining AKT activation even in the presence of a constitutively activated PI3K pathway. The treatment of TALL models with this compound significantly increases the Phlda3 mRNA and protein levels, accompanied by substantial decreases in the levels of P-AKT and P-S6 as well as the PPP metabolic intermediates. |
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































