- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Tolbutamide (HLS 831) Potassium Channel inhibitor
Cat.No.S2443
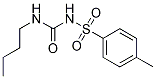
Chemical Structure
Molecular Weight: 270.35
Jump to
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Sodium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Potassium Channel Products | Nicorandil TRAM-34 Sophocarpine ML133 HCl Hydralazine HCl Gliquidone PAP-1 VU0071063 Apamin A2793 |
Solubility
|
In vitro |
DMSO
: 54 mg/mL
(199.74 mM)
Ethanol : 54 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 270.35 | Formula | C12H18N2O3S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 64-77-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCCCNC(=O)NS(=O)(=O)C1=CC=C(C=C1)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Potassium channel
|
|---|---|
| In vitro |
Tolbutamide belongs to a class of medications called sulfonylureas. Tolbutamide lowers blood sugar by causing the pancreas to produce insulin (a natural substance that is needed to break down sugar in the body) and helping the body use insulin efficiently. This medication will only help lower blood sugar in people whose bodies produce insulin naturally. Tolbutamide is not used to treat type 1 diabetes (condition in which the body does not produce insulin and, therefore, cannot control the amount of sugar in the blood) or diabetic ketoacidosis (a serious condition that may occur if high blood sugar is not treated). Tolbutamide inhibits both the basal and the cyclic AMP-stimulated protein kinase activities and the IC50 of Tolbutamide is 4 mM. Similar Tolbutamide concentrations are required for half maximal inhibition of in vitro lipolysis induced by hormones (norepinephrine and ACTH) or by dibutyryl cyclic AMP plus theophylline. Tolbutamide also inhibits both soluble and membrane-bound protein kinase from canine heart. The Tolbutamide inhibition of adipose tissue cyclic AMP-dependent protein kinase is one possible explanation for the antilipolytic effects of this drug. Tolbutamide inhibits C6-glioma cell proliferation by increasing Cx43, which correlates with a reduction in pRb phosphorylation due to the up-regulation of the Cdk inhibitors p21 and p27. Cytosolic nucelotides enhance the Tolbutamide sensitivity of the ATP-dependent K+ channel in mouse pancreatic B cells by their combined actions at inhibitory and stimulatory receptors. Tolbutamide inhibits glucagon-induced phosphorylation of the bifunctional enzyme protein in a dose-dependent manner. By adding 2 mM Tolbutamide, reduces activity of 6PF-2-K and increased activity of Fru-2,6-P2ase in the presence of 10(-9) M glucagon are partially restored. The present results suggest the possibility that Tolbutamide modulates the activity of hepatic 6PF-2-K/Fru-2,6-P2ase through inhibiting a phosphorylation of the enzyme protein.
|
| Kinase Assay |
cAMP kinase assay
|
|
Diced epididymal fat pads from fed Wistar rats (175-225 gm) are obtained after decapitation and incubated at 37 °C for two hours in Krebs-bicarbonate buffer containing 1.27 mM CaCl2. When added, Tolbutamide is present only during the incubation. After incubation fat pads are rinsed and sonicated in cold Krebs-bicarbonate buffer. The aqueous supematants from centrifugation at 50,000 × g for 30 minutes at 4 °C contained 0.75 to 1.25 mg protein per mL and are assayed for cyclic AMP-stimulated protein kinase activity. The assay is performed in 0.2 mL with these additions, 10 μmoles sodium glycerofiosphate pH 7.0, 2 μmoles sodium fluoride, 0.4 μmoles theophylline, 0.1 μmoles ethylene glyool bis (β-aminoethyl ether)-N, N'-tetraaoetic acid, 3 μmoles magnesium chloride, 0.3 mg mixed histone, 2 nmoles (γ- 32P) ATP, 1 nmoles cyclic AMP when indicated, and 0.05 ml of supernatant.
|
|
| In vivo |
450 mg Tolbutamide/kg/day given for 7 days significantly increases the binding of insulin to isolated adipocytes. The binding curves reflect an increase in the number of receptor sites rather than in the affinity. The effect is associated with an enhanced response to insulin of the adipose tissue, since the fat cells obtained from animals treated with Tolbutamide convert significantly more glucose to lipids in the presence of insulin than those obtained from the control group. However, the augmentation of insulin binding sites is observed only at a large tolbutamide dosage, which reduces the pancreatic insulin content, the secretory response of the isolated pancreas, and the serum insulin levels. Smaller doses, sufficient to produce metabolic effects via a stimulation of insulin secretion, do not provide additional insulin binding sites.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05830799 | Completed | Drug Interaction |
Vicore Pharma AB |
March 29 2023 | Phase 1 |
| NCT05097716 | Completed | Healthy Volunteers |
Pfizer |
November 2 2021 | Phase 1 |
| NCT03291288 | Completed | Drug Interaction Potential |
Daiichi Sankyo |
February 26 2018 | Phase 1 |
| NCT02819102 | Completed | Hereditary Angioedema |
BioCryst Pharmaceuticals |
March 2016 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































