research use only
Larotrectinib sulfate Trk receptor inhibitor
Cat.No.S7960
Chemical Structure
Molecular Weight: 526.51
Quality Control
| Related Targets | EGFR VEGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 |
|---|---|
| Other Trk receptor Inhibitors | ANA-12 GW441756 7,8-Dihydroxyflavone GNF-5837 Selitrectinib (LOXO-195) Altiratinib N-Acetyl-5-hydroxytryptamine LM22A-4 CH7057288 PF-06273340 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| KM12 cells | Function assay | 0, 10, 100, 1000 nM | 3 hours | LOXO-101 inhibited NTRK1 phosphorylation at 100 nmol/L | 28751539 | |
| Ba/F3 | Function assay | 0, 10, 100, 1000 nM | 3 hours | LOXO-101 inhibited NTRK1 phosphorylation at 100 nmol/L, | 28751539 | |
| CR20 | Function assay | 0, 10, 100, 1000 nM | 3 hours | NTRK1 phosphorylation was inhibited | 28751539 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(189.92 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 526.51 | Formula | C21H22F2N6O2.H2SO4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1223405-08-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LOXO-101 sulfate, ARRY-470 sulfate | Smiles | C1CC(N(C1)C2=NC3=C(C=NN3C=C2)NC(=O)N4CCC(C4)O)C5=C(C=CC(=C5)F)F.OS(=O)(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
TRK
|
|---|---|
| In vitro |
ARRY-470(LOXO-101) is a selective kinase inhibitor with nanomolar activity against TRKA, TRKB and TRKC but no other notable kinase inhibition below 1,000 nM. ARRY-470 does not inhibit proliferation of Ba/F3 cells expressing other oncogene targets (epidermal growth factor receptor (EGFR), ALK or ROS1) or of lung and colorectal cell lines that do not harbor an NTRK1 fusion. It induces cell-cycle arrest in G1 and apoptosis of KM12 cells.
|
| In vivo |
Early/sustained but not late/acute administration of ARRY-470(LOXO-101) markedly attenuates bone cancer pain and significantly blocks the ectopic sprouting of sensory nerve fibers and the formation of neuroma-like structures in the tumor bearing bone, but does not have a significant effect on tumor growth or bone remodeling. It has very limited ability crossing of the blood brain barrier.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pTRKA / TRKA / pTRKC / TRKC / p-AKT / AKT p-NTRK(Y490) / p-ERK (T202/Y204) / p-Eif4e |
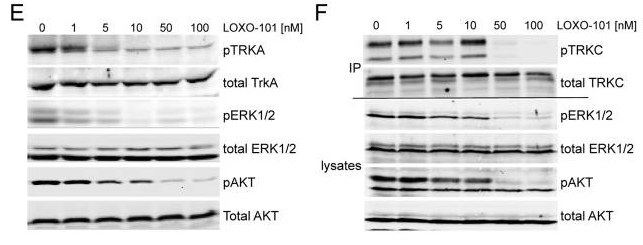
|
26216294 |
| Growth inhibition assay | Cell proliferation |
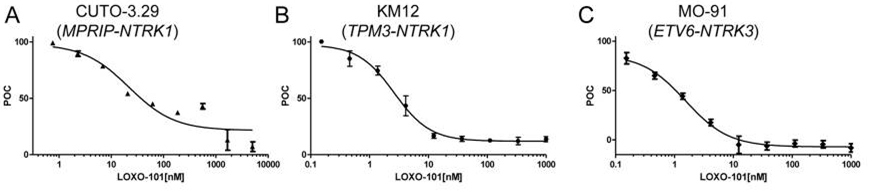
|
26216294 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04655404 | Recruiting | High Grade Glioma|Diffuse Intrinsic Pontine Glioma |
Nationwide Children''s Hospital |
April 8 2021 | Early Phase 1 |
| NCT04814667 | Active not recruiting | Solid Tumor Adult|Locally Advanced Solid Tumor|Metastatic Cancer |
Centre Leon Berard|Bayer |
February 23 2021 | -- |
| NCT04142437 | Recruiting | Locally Advanced or Metastatic Solid Tumor Harboring an NTRK Gene Fusion |
Bayer |
April 3 2020 | -- |
| NCT03834961 | Active not recruiting | Central Nervous System Neoplasm|Infantile Fibrosarcoma|Recurrent Acute Leukemia|Refractory Acute Leukemia|Solid Neoplasm |
Children''s Oncology Group|National Cancer Institute (NCI) |
October 25 2019 | Phase 2 |
| NCT02637687 | Active not recruiting | Solid Tumors Harboring NTRK Fusion |
Bayer |
December 16 2015 | Phase 1|Phase 2 |
| NCT02576431 | Active not recruiting | Solid Tumors Harboring NTRK Fusion |
Bayer |
September 30 2015 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































