- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
Alectinib ALK inhibitor
Alectinib is a potent ALK inhibitor with IC50 of 1.9 nM in cell-free assays, sensitive to L1196M mutation and higher selectivity for ALK than PF-02341066, NVP-TAE684 and PHA-E429.
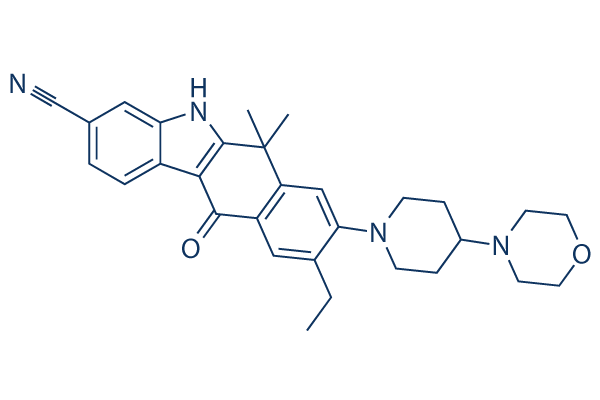
Chemical Structure
Molecular Weight: 482.62
Purity & Quality Control
Batch:
Purity:
99.95%
99.95
Related Products
| Related Products | TAE684 (NVP-TAE684) GSK1838705A Repotrectinib (TPX-0005) AZD3463 AP26113-analog (ALK-IN-1) Ensartinib dihydrochloride ASP3026 | Click to Expand |
|---|---|---|
| Related Compound Libraries | Kinase Inhibitor Library Tyrosine Kinase Inhibitor Library PI3K/Akt Inhibitor Library Cell Cycle compound library Angiogenesis Related compound Library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| NCI-H2228 | Kinase assay | ~1 μM | prevents autophosphorylation of ALK | 21575866 | ||
| KARPAS-299 | Growth inhibitory assay | ~10 μM | IC50=3 nM | 21575866 | ||
| SR | Growth inhibitory assay | ~10 μM | IC50=6.9 nM | 21575866 | ||
| HDLM-2 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| NB-1 | Growth inhibitory assay | ~10 μM | IC50=4.5 nM | 21575866 | ||
| KELLY | Growth inhibitory assay | ~10 μM | IC50=62 nM | 21575866 | ||
| SK-N-FI | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| NCI-H2228 | Growth inhibitory assay | ~10 μM | IC50=53 nM | 21575866 | ||
| Calu-3 | Growth inhibitory assay | ~10 μM | IC50=>10,000 nM | 21575866 | ||
| PC-1 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| NCI-H23 | Growth inhibitory assay | ~10 μM | IC50=3600 nM | 21575866 | ||
| Calu-1 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| NCI-H2009 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| NCI-H1993 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| MKN-45 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| SNU-5 | Growth inhibitory assay | ~10 μM | IC50=1800 nM | 21575866 | ||
| KATO-III | Growth inhibitory assay | ~10 μM | IC50=7900 nM | 21575866 | ||
| SK-BR-3 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| BT-483 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| PC-3 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| 22Rv1 | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| U-87 MG | Growth inhibitory assay | ~10 μM | IC50>10,000 nM | 21575866 | ||
| H3122 | Growth inhibitory assay | ~10 μM | IC50=33 nM | 25096400 | ||
| LC-2/ad | Apoptosis assay | ~1 μM | DMSO | induces apoptosis | 25349307 | |
| LC-2/ad | Function assay | ~1 μM | DMSO | inhibits the MAPK signaling pathway | 25349307 | |
| Ba/F3 | Function assay | ~1 μM | DMSO | suppresses phosphorylation of ERK and increases the abundance of BIM | 25349307 | |
| SNU-2535 | Growth inhibitory assay | ~10 μM | IC50=33.1 nM | 26849637 | ||
| SNU-2535 | Kinase assay | ~1 μM | inhibits the phosphorylation of ALK and its downstream molecules ERK1/2 and AKT | 26849637 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK C1156Y mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.002 μM. | 26568289 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK S1206Y mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.002 μM. | 26568289 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of wild type EML4-ALK (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.002 μM. | 26568289 | ||
| KARPAS299 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human KARPAS299 cells after 96 hrs by cell counting assay, IC50 = 0.003 μM. | 22225917 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK F1174L mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.003 μM. | 26568289 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK G1269A mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.009 μM. | 26568289 | ||
| NCI-H3122 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H3122 cells after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.009 μM. | 26568289 | ||
| KARPAS299 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KARPAS299 cells after 72 hrs by SRB/CCK-8 assay, IC50 = 0.015 μM. | 27131066 | ||
| NCI-H3122 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H3122 cells after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0174 μM. | 27131066 | ||
| SUP-M2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SUP-M2 cells after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0179 μM. | 27131066 | ||
| NCI-H3122 | Function assay | 72 hrs | Inhibition of ALK expressed in human NCI-H3122 cells assessed as cell growth inhibition after 72 hrs by SRB/CCK-8 assay, IC50 = 0.019 μM. | 27131066 | ||
| NCI-H3122 | Antiproliferative assay | 72 hrs | Antiproliferative activity against ALK-dependent human NCI-H3122 cells after 72 hrs, IC50 = 0.019 μM. | 26476749 | ||
| SU-DHL1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SU-DHL1 cells after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0205 μM. | 27131066 | ||
| NIH/3T3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse NIH/3T3 cells expressing wild type EML4-ALK after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0323 μM. | 27131066 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK 1151Tins mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.072 μM. | 26568289 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK L1196M mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.09 μM. | 26568289 | ||
| NIH/3T3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse NIH/3T3 cells expressing EML4-ALK L1196 mutant after 72 hrs by SRB/CCK-8 assay, IC50 = 0.132 μM. | 27131066 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK L1152R mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.169 μM. | 26568289 | ||
| Ba/F3 | Function assay | 72 hrs | Inhibition of EML4-ALK G1202R mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.207 μM. | 26568289 | ||
| DFCI114 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DFCI114 cells expressing EML4-ALK G1269A mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.207 μM. | 26568289 | ||
| CHLA20 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CHLA20 cells expressing EML4-ALK R1275Q mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.43 μM. | 26568289 | ||
| Kelly | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Kelly cells expressing EML4-ALK F1174L mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.434 μM. | 26568289 | ||
| DFCI76 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DFCI76 cells expressing EML4-ALK L1152R mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.511 μM. | 26568289 | ||
| LAN5 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LAN5 cells expressing EML4-ALK R1275Q mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.617 μM. | 26568289 | ||
| SMS-KCNR | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SMS-KCNR cells expressing EML4-ALK R1275Q mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.765 μM. | 26568289 | ||
| SK-N-SH | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-SH cells expressing EML4-ALK F1174L mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 0.872 μM. | 26568289 | ||
| SH-SY5Y | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SH-SY5Y cells expressing EML4-ALK F1174L mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 1.15 μM. | 26568289 | ||
| SK-N-BE(2) | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-BE(2) cells expressing wild type EML4-ALK after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 1.554 μM. | 26568289 | ||
| LAN1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LAN1 cells expressing EML4-ALK F1174L mutant after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 2.004 μM. | 26568289 | ||
| SK-N-AS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-AS cells expressing wild type EML4-ALK after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 2.139 μM. | 26568289 | ||
| SK-N-FI | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-FI cells expressing wild type EML4-ALK after 72 hrs by CellTiter-Glo Luminescent Cell Viability Assay, EC50 = 2.401 μM. | 26568289 | ||
| NIH/3T3 | Antitumor assay | 50 mg/kg | 10 days | Antitumor activity against mouse NIH/3T3 cells expressing EML4-ALK L1196M mutant xenografted in nude mouse assessed as tumor stasis at 50 mg/kg, po qd administered for 10 days | 27131066 | |
| NIH/3T3 | Antitumor assay | 50 mg/kg | 10 days | Antitumor activity against mouse NIH/3T3 cells expressing EML4-ALK L1196M mutant xenografted in nude mouse assessed as partial tumor regression at 50 mg/kg, po qd administered for 10 days | 27131066 | |
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Targets |
|
|---|
In vitro |
||||
| In vitro | The dissociation constant (KD) value of CH5424802 for ALK in an ATP-competitive manner is 2.4 nM. CH5424802 has substantial inhibitory potency against both native ALK and L1196M with Ki of 0.83 nM and 1.56 nM, respectively. CH5424802 prevents autophosphorylation of ALK in NCI-H2228 NSCLC cells expressing EML4-ALK. CH5424802 also suppresses the phosphorylation of STAT3 and AKT, but not of ERK1/2. CH5424802 completely inhibits the phosphorylation of STAT3 at Tyr705. CH5424802 is preferentially efficacious against NCI-H2228 cells expressing EML4-ALK, but not ALK fusion-negative NSCLC cell lines, including HCC827 cells (EGFR exon 19 deletion), A549 cells (KRAS mutant), or NCI-H522 cells (EGFR wild-type, KRAS wild-type, and ALK wild-type) in monolayer culture. CH5424802 elicits an apoptotic marker—caspase-3/7-like activation—in NCI-H2228 spheroid cells. CH5424802 blocks the growth of two lymphoma lines, KARPAS-299 and SR, with NPM-ALK fusion protein but does not influence the growth of an HDLM-2 lymphoma line without ALK fusion. [1] CH5424802 displays high target selectivity and the stronger anti-proliferative activity against KARPAS-299. CH5424802 inhibits KAPRAS-299 with an IC50 of 3 nM, and KDR with IC50 of 1.4 μM. The metabolic stability of CH5424802 is very high.[2] | |||
|---|---|---|---|---|
| Kinase Assay | Kinase inhibitory assays in Vitro | |||
| The inhibitory ability against each kinase except for MEK1 and Raf-1 is evaluated by examining their ability to phosphorylate various substrate peptides in the presence of CH5424802 using time-resolved fluorescence resonance energy transfer (TR-FRET) assay or fluorescence polarization (FP) assay. The inhibitory activity against MEK1 is evaluated by quantitative analysis of the phosphorylation of a substrate peptide by a recombinant ERK2 protein in the presence of CH5424802. The inhibitory activity against Raf-1 is evaluated by examining the ability of the kinases to phosphorylate MEK1 in the presence of CH5424802. | ||||
| Cell Research | Cell lines | NSCLC, A549 and HCC827 cell lines | ||
| Concentrations | 0-1 μM | |||
| Incubation Time | 5 days | |||
| Method | Cells including NSCLC, A549 and HCC827 are seeded in 96-well plates overnight and incubated with various concentrations of CH5424802 for the indicated time. For spheroid cell growth inhibition assay, cells are seeded on spheroid plates, incubated overnight, and then treated with compound for the indicated times. The viable cells are measured by the Luminescent Cell Viability Assay. Caspase-3/7 assay is evaluated using the Caspase-Glo 3/7 Assay Kit. | |||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | pALK / ALK / pAKT / AKT / pERK / ERK / pS6 / S6 PARP / cleaved PARP / Akt / caspase 3 / Cleaved caspase 3 pROS1 / ROS1 / pSTAT3 / STAT3 p-EGFR Tyr1068 / EGFR / p-HER3 Tyr1222 / HER3 / p-IGF-1R Tyr1135 / IGF-1R |
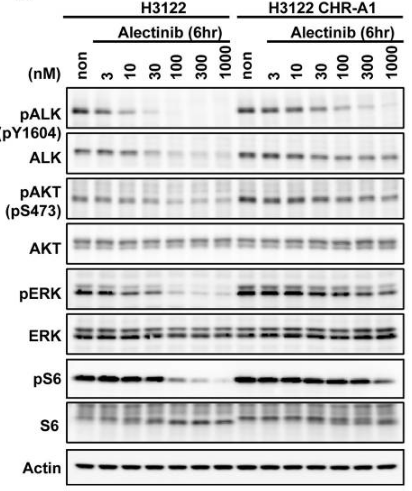
|
25228534 | |
| Growth inhibition assay | Cell viability |
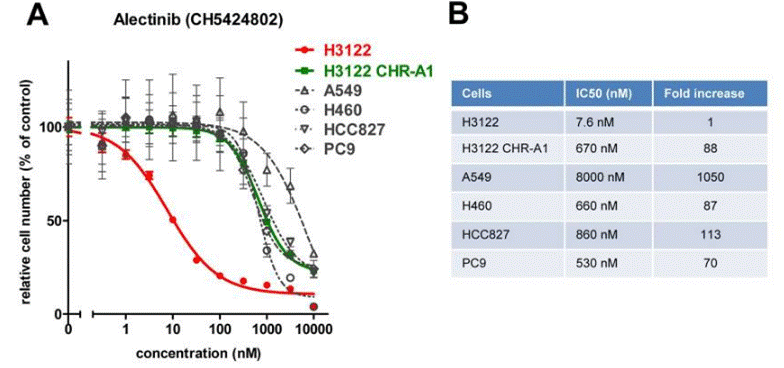
|
25228534 | |
In Vivo |
||
| In vivo | Oral administration of CH5424802 dose-dependently inhibits tumor growth with an ED50 of 0.46 mg/kg and tumor regression. Treatment of 20 mg/kg CH5424802 reveals rapid tumor regression by 168%, the tumor volume in any mouse is <30 mm3 after 11 days of treatment (at day 28), a potent antitumor effect is maintained, and tumor regrowth does not occur throughout the 4-week drug-free period. The half-life and the oral bioavailability of CH5424802 in mice are 8.6 hours and 70.8%, respectively. At a repeated dose of 6 mg/kg, the mean plasma levels reached 1.7, 1.5, and 0.3 nM at 2, 7, and 24 hours post-dose, respectively. Administration of CH5424802 leads to tumor growth prevention and tumor regression. Tumor growth inhibition at 20 mg/kg is 119% for KARPAS-299 and 104% for NB-1 on day 20. CH5424802 inhibits the phosphorylation of STAT3 in a dose-dependent manner (2–20 mg/kg). A partial decrease in AKT phosphorylation is also observed in CH5424802-treated xenograft tumors. [1] | |
|---|---|---|
| Animal Research | Animal Models | SCID or nude mice bearing NCI-H2228 |
| Dosages | 20 mg/kg | |
| Administration | Oral administration | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05987956 | Not yet recruiting | Non-small Cell Lung Cancer |
Han Xu M.D. Ph.D. FAPCR Sponsor-Investigator IRB Chair|Medicine Invention Design Inc |
March 8 2024 | Phase 2|Phase 3 |
| NCT05987644 | Recruiting | Lung Cancer|NSCLC|Brain Metastases |
Joshua Palmer|Genentech Inc.|Hoosier Cancer Research Network |
March 7 2024 | Phase 1|Phase 2 |
| NCT05710133 | Enrolling by invitation | NSCLC |
The Netherlands Cancer Institute |
February 1 2024 | Not Applicable |
| NCT05770037 | Recruiting | Solid Tumor|Haematological Malignancy|Malignant Neoplasm|Lymphoproliferative Disorders|Neoplasms by Histologic Type|Neoplasms by Site|Cancer|Anaplastic Large Cell Lymphoma|Lymphoma|Renal Cell Carcinoma|Neuroblastoma |
Cancer Research UK|University of Manchester|University of Birmingham|Royal Marsden NHS Foundation Trust|Hoffmann-La Roche |
December 18 2023 | Phase 2|Phase 3 |
| NCT05525338 | Recruiting | Drug Monitoring|Carcinoma Non-Small-Cell Lung|Lung Cancer|Anaplastic Lymphoma Kinase Gene Mutation|Anaplastic Lymphoma Kinase Gene Translocation |
University Medical Center Groningen|Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Erasmus Medical Center|Maastricht University Medical Center|Radboud University Medical Center|The Netherlands Cancer Institute|Leiden University Medical Center |
March 23 2022 | Phase 4 |
| NCT04774718 | Recruiting | ALK Fusion-positive Solid or CNS Tumors |
Hoffmann-La Roche |
September 14 2021 | Phase 1|Phase 2 |
References |
|
Chemical Information
| Molecular Weight | 482.62 | Formula | C30H34N4O2 |
| CAS No. | 1256580-46-7 | SDF | Download SDF |
| Synonyms | AF-802, RG-7853,CH5424802 | ||
| Smiles | CCC1=CC2=C(C=C1N3CCC(CC3)N4CCOCC4)C(C5=C(C2=O)C6=C(N5)C=C(C=C6)C#N)(C)C | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 7 mg/mL ( (14.5 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field