- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Birinapant (TL32711) IAP antagonist
Cat.No.S7015
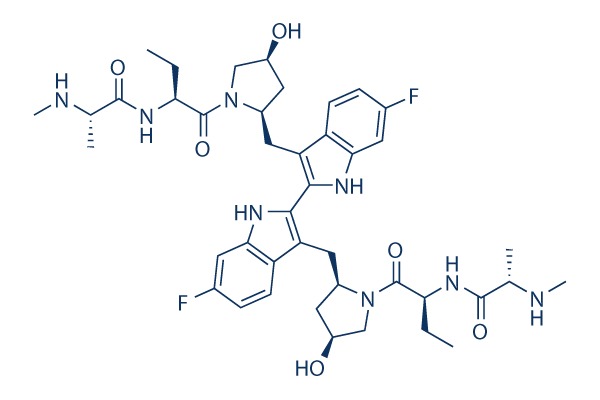
Chemical Structure
Molecular Weight: 806.94
Jump to
Quality Control
Batch:
Purity:
99.98%
99.98
Products Often Used Together with Birinapant (TL32711)
| Related Targets | Bcl-2 Caspase K-Ras PD-1/PD-L1 Ferroptosis p53 Apoptosis related Synthetic Lethality STAT TNF-alpha |
|---|---|
| Other IAP Inhibitors | SM-164 BV6 LCL161 Xevinapant (AT406) GDC-0152 AZD5582 Tolinapant (ASTX660) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| PANC-1 | Growth Inhibition Assay | 50/200/500 nM | 0-96 h | inhibits cell growth in both time and dose dependent manner | 26252969 | |
| Molm13 | Function Assay | 2/20/200 nM | 24 h | decreases cIAP1 and, to a much lesser extent, cIAP2, and XIAP under various conditions | 24526787 | |
| WTH202 | Growth Inhibition Assay | 72 h | IC50=1.8 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM793B | Growth Inhibition Assay | 72 h | IC50=2.5 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM9 | Growth Inhibition Assay | 72 h | IC50=2.4 nM | 23403634 | ||
| WM9 | Growth Inhibition Assay | 72 h | IC50=2.7 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM1366 | Growth Inhibition Assay | 72 h | IC50=7.9 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM164 | Growth Inhibition Assay | 72 h | IC50=9 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| 451Lu | Growth Inhibition Assay | 72 h | IC50=14.2 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM1341D | Growth Inhibition Assay | 72 h | IC50=57.6 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM3130 | Growth Inhibition Assay | 72 h | IC50=64.3 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM1985 | Growth Inhibition Assay | 72 h | IC50=97 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| WM3854 | Growth Inhibition Assay | 72 h | IC50=226 nM, combined with 1ng/ml TNF-α | 23403634 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against cIAP/XIAP-dependent human MDA-MB-231 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.01 μM. | 25584393 | ||
| A875 | Cytotoxicity assay | 72 hrs | Cytotoxicity against XIAP-dependent human A875 cells assessed as cell viability after 72 hrs by MTS assay, IC50 = 0.04 μM. | 25584393 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(123.92 mM)
Ethanol : 55 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 806.94 | Formula | C42H56F2N8O6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1260251-31-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | TL32711 | Smiles | CCC(C(=O)N1CC(CC1CC2=C(NC3=C2C=CC(=C3)F)C4=C(C5=C(N4)C=C(C=C5)F)CC6CC(CN6C(=O)C(CC)NC(=O)C(C)NC)O)O)NC(=O)C(C)NC | ||
Mechanism of Action
| Targets/IC50/Ki |
cIAP1
(Cell-free assay) <1 nM(Kd)
XIAP
(Cell-free assay) 45 nM(Kd)
|
|---|---|
| In vitro |
Birinapant binds with XIAP and cIAP1 with Kd of 45 and <1 nM, respectively. This compound induces cell death as a single agent in TRAIL-insensitive SUM190 (ErbB2-overexpressing) cells (IC50, ~300 nM), and significantly increases potency of TRAIL-induced apoptosis in TRAIL-sensitive SUM149 (triple-negative, EGFR-activated) cells. It causes rapid cIAP1 degradation, caspase activation, PARP cleavage, and NF-κB activation.
This chemical in combination with TNF-α exhibits a strong antimelanoma effect in vitro. It in combination with TNF-α(1 ng/mL) inhibits the growth of human melanoma cell lines WTH202, WM793B, WM1366 and WM164 with IC50s of 1.8, 2.5, 7.9 and 9 nM, respectively, while neither compound is effective individually. This compound singly treatment induces inhibition on proliferation of WM9 cells with IC50 of 2.4 nM. It significantly inhibits the target protein cIAP1 and cIAP2 in these cell lines.
|
| Kinase Assay |
Fluorescence polarization assay
|
|
The binding affinities of compounds to XIAP and cIAP1 are determined using a fluorogenic substrate and are reported as Kd values. Initially, the dissociation constant (Kd) for the fluorescently labeled modified Smac peptide (AbuRPF-K(5-Fam)-NH2; FP pep-tide) is determined using a fixed concentration of peptide (5 nM) and titrating varying concentrations of protein (0.075–5 μM in half log dilutions). The dose–response curves are produced by a nonlinear least squares fit to a single-site binding model using GraphPad Prism, with 5 nM of FP peptide and 50 nM of XIAP used in the assay. Various concentrations of Smac mimetics (100–0.001 μM in half log dilutions) are added to FP peptide:protein binary complex for 15 min at room temperature in 100μL of 0.1 M potassium phosphate buffer, pH 7.5, containing 100 mg/mL bovine c -globulin. Following incubation, the polarization values are measured on a multi-label plate reader using a 485 nm excitation filter and a 520 nm emission filter.
|
|
| In vivo |
Birinapant (30 mg/kg) treatment significantly induces abrogation of tumor growth in melanoma xenotransplantation models 451Lu with.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | cIAP1 / cIAP2 / XIAP NF-κB(p65) / IκBa / Bcl-xl / NF-κB(p100) / p52 BIRC2 / ARC |

|
24526787 |
| Immunofluorescence | Caspase 3/7 |
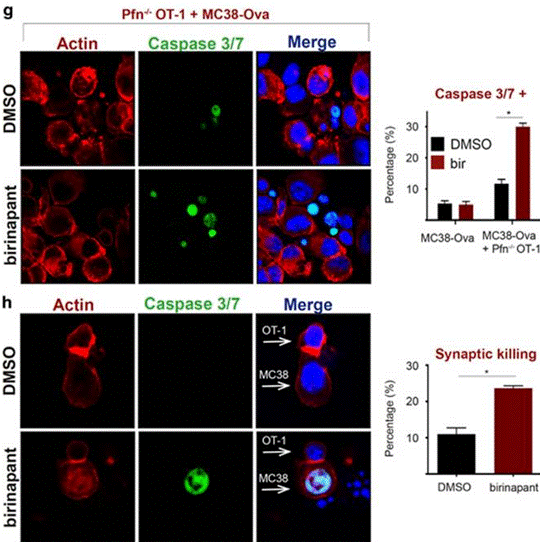
|
28665401 |
| Growth inhibition assay | Cell viability |
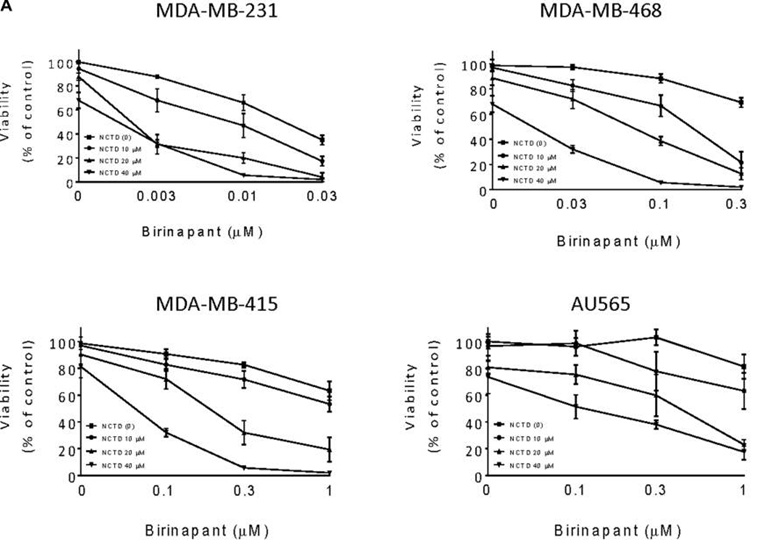
|
28460471 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01681368 | Terminated | Epithelial Ovarian Cancer|Peritoneal Neoplasms|Fallopian Tube Neoplasms |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
August 15 2012 | Phase 2 |
| NCT01486784 | Terminated | Acute Myelogenous Leukemia |
Abramson Cancer Center at Penn Medicine |
November 2011 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































